Page 207 - 2021_05-Haematologica-web
P. 207
Effects of absence of PTGS1 in platelets
The 965G>C variant of PTGS1 found in the family reported here is absent from gnomAD. In the identified pedigree, however, three of the eight family members studied were homozygous due to consanguinity and three were heterozygous for the variant. Interestingly, since this is a missense variant outside both the functional sites of the COX enzyme the phenotype was unexpected. Within the homozygous carriers, despite similar reproducible platelet aggregation, we saw minor differences in the
bleeding phenotype, which reflects the clinical hetero- geneity of presentation of some of the rare platelet disor- ders. This is also consistent with observations that while millions of people take aspirin daily to prevent secondary cardiovascular events, and this increases their risk of bleeding, the vast majority do not suffer from major spon- taneous bleeding. Similarly, mice with a deficiency in COX-1 exhibit impaired haemostasis but only after being challenged by the tail-bleeding assay.24,25
A
B
CD
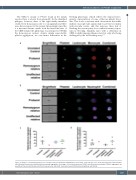
Figure 3. Analysis of unstimulated whole blood acquired using an ImageStreamX Mark II incorporating a 60x objective lens. Scale bars represent 7 μm and iden- tified (A) platelet-monocyte and (B) platelet-neutrophil aggregates. Percentage of (C) platelet-monocyte (CD14+) and (D) platelet-neutrophil (CD66b+) aggregates are quantified. Platelets identified by anti-CD61, leukocytes by anti-CD45, monocytes by anti-CD14 and neutrophils by anti-CD66b.
haematologica | 2021; 106(5)
1427