Page 258 - Haematologica - Vol. 105 n. 6 - June 2020
P. 258
Y. Lu et al.
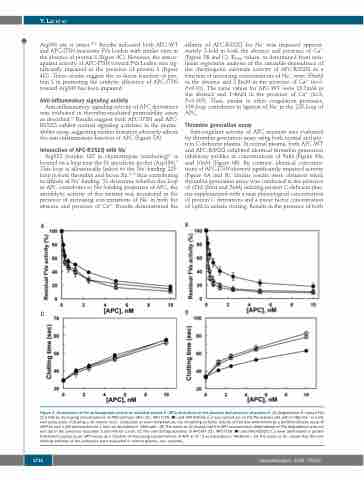
Arg306 site is intact.29,30 Results indicated both APC-WT and APC-I73N inactivate FVa Leiden with similar rates in the absence of protein S (Figure 4C). However, the antico- agulant activity of APC-I73N toward FVa Leiden was sig- nificantly impaired in the presence of protein S (Figure 4D). These results suggest the co-factor function of pro- tein S in promoting the catalytic efficiency of APC-I73N toward Arg306 has been impaired.
Anti-inflammatory signaling activity
Anti-inflammatory signaling activity of APC derivatives was evaluated in thrombin-mediated permeability assay as described.24 Results suggest both APC-I73N and APC- R352Q exhibit normal signaling activities in the perme- ability assay, suggesting neither mutation adversely affects the anti-inflammatory function of APC (Figure 5A).
Interaction of APC-R352Q with Na+
Arg352 (residue 187 in chymotrypsin numbering)21 is located on a loop near the S1 specificity pocket (Asp189).5 This loop is allosterically linked to the Na+-binding 225- loop in both thrombin and factor Xa,31-33 thus contributing to affinity of Na+ binding. To determine whether this loop in APC contributes to Na+-binding properties of APC, the amidolytic activity of this mutant was monitored in the presence of increasing concentrations of Na+ in both the absence and presence of Ca2+. Results demonstrated the
A
affinity of APC-R352Q for Na+ was impaired approxi- mately 2-fold in both the absence and presence of Ca2+ (Figure 5B and C). Kd(app) values, as determined from non- linear regression analysis of the saturable-dependence of the chromogenic substrate activity of APC-R352Q as a function of increasing concentrations of Na+, were 35mM in the absence and 2.8mM in the presence of Ca2+ (n=3, P<0.01). The same values for APC-WT were 18.7mM in the absence and 1.4mM in the presence of Ca2+ (n=3, P<0.005). Thus, similar to other coagulation proteases, 189-loop contributes to ligation of Na+ in the 225-loop of APC.
Thrombin generation assay
Anticoagulant activity of APC mutants was evaluated by thrombin generation assay using both normal and pro- tein C-deficient plasma. In normal plasma, both APC-WT and APC-R352Q exhibited identical thrombin generation inhibitory profiles at concentrations of 5nM (Figure 6A) and 10nM (Figure 6B). By contrast, identical concentra- tions of APC-I73N showed significantly impaired activity (Figure 6A and B). Similar results were obtained when thrombin generation assay was conducted in the presence of sTM (2nM and 5nM) utilizing protein C-deficient plas- ma supplemented with a near physiological concentration of protein C derivatives and a tissue factor concentration of 1pM to initiate clotting. Results in the presence of both
B
C
D
Figure 3. Assessment of the anticoagulant activity of activated protein C (APC) derivatives in the absence and presence of protein S. (A) Degradation of human FVa (2.5 nM) by increasing concentrations of APC-wild type (WT) (), APC-I73N () and APC-R352Q () was carried out on PC/PS vesicles (25 μM) in TBS/Ca2+ in a 96- well assay plate. Following a 10-minute (min) incubation at room temperature, the remaining co-factor activity of FVa was determined by a prothrombinase assay (5 nM FXa and 1 μM prothrombin for 1 min) as described in “Methods”. (B) The same as (A) except that the APC concentration dependence of FVa degradation was car- ried out in the presence of protein S (50 nM) for 1 min. (C) The anti-clotting activities of APC-WT (), APC-I73N () and APC-R352Q () were determined in protein S-deficient plasma by an aPTT assay as a function of increasing concentrations of APC at 37°C as described in “Methods”. (D) The same as (C) except that the anti- clotting activities of the proteases were evaluated in normal plasma. sec: seconds.
1716
haematologica | 2020; 105(6)