Page 256 - Haematologica - Vol. 105 n. 6 - June 2020
P. 256
Y. Lu et al.
tives were separated from thrombin on the Mono Q col- umn as described.22 Concentrations of APC derivatives, as determined by active-site titration, were within 90-100% of those expected based on zymogen concentrations as determined from absorbance at 280 nm. SDS-PAGE analy- sis indicated APC derivatives have been purified to near homogeneity, with the heavy chains of both APC-I73N and APC-R352Q migrating as triple bands representing α, β and γ subforms of protein C (Figure 2A), which are gly- cosylation variants of the protein also observed in APC- WT.26 Triplet bands of APC-I73N migrated as diffused bands, but slower than those observed with APC-WT and APC-R352Q (Figure 2A). SDS-PAGE analysis under reduc- ing conditions revealed the higher molecular mass of APC- I73N under non-reducing conditions is due to its light chain, which migrated slower than light chains of other two APC derivatives (Figure 2A). As expected, heavy chains of all three APC derivatives migrated with near similar apparent molecular masses under reducing condi- tions (Figure 2A). Analysis of the cDNA sequence of pro- tein C revealed that Ile73 to Asn mutation creates a poten- tial new N-linked glycosylation (73Asn-Gly-Ser75) site on EGF1-domain of protein C, suggesting the glycosylation of this mutant residue is responsible for the higher molec- ular mass of the protein (Figure 2A). In agreement with this observation, treatment with PNGase F, which cat- alyzes the cleavage of N-linked oligosaccharides, eliminat- ed the diffused nature of bands as well as differences in molecular masses observed between APC-WT and APC- I73N (Figure 2A, right). As expected, SDS-PAGE analysis of WT and I73N protein C zymogens indicated both pro-
AB
teins also migrate as triplet bands representing α, β and γ subforms of protein C and similar to APC derivatives, I73N zymogen migrated slower than WT protein under non-reducing conditions (Figure 2C). Analysis of zymogen derivatives under reducing conditions indicated a fraction of both proteins (a larger fraction with I73N) is not processed at the dibasic cleavage site,27 thus migrating as single chains (Figure 2C). The single chain I73N zymogen migrated much slower than the single chain WT under reducing conditions (Figure 2C), most likely due to the additional glycan chain in the light chain of the mutant protein. Previously, we have demonstrated the single chain APC has normal amidolytic and anticoagulant activ- ity.27 Noting the compound heterozygous nature of pro- tein C mutation, the relative contribution of each muta- tion to lower PC:Ag in the proband’s plasma remains unknown.
Amidolytic activity of APC derivatives toward SpPCa is presented in Figure 2D. Kinetic analysis indicated APC- WT, APC-I73N, and APC-R352Q cleave SpPCa with sim- ilar kinetic parameters in TBS/Ca2+ (Km(app)=220-240μM and kcat=19-22s-1), suggesting neither mutation has an adverse effect on folding and/or reactivity of the catalytic pocket.
Analysis of protein C activation
Analysis of the initial rate of protein C activation indi- cated relative to WT, thrombin activates both variants with similar or improved rates. Thus, in EDTA containing TBS, the rate of protein C activation by thrombin was improved 1.5- and 2-fold for I73N and R352Q, respective- ly (Figure 2E). However, the initial rate of activation by
C
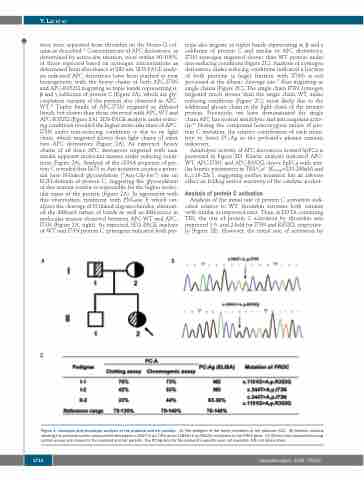
Figure 1. Genotype and phenotype analysis of the proband and her parents. (A) The pedigree of the family members of the proband (II-2). (B) Genetic analysis showing the proband carries compound heterozygous c.344T>A (p.I73N) and c.1181G>A (p.R352Q) mutations in the PROC gene. (C) Clinical data obtained by coag- ulation assays are shown for the proband and her parents. The PC:Ag data for the proband's parents were not available. ND: not determined.
1714
haematologica | 2020; 105(6)