Page 259 - Haematologica - Vol. 105 n. 6 - June 2020
P. 259
APC-I73N variant
concentrations of sTM confirmed the anticoagulant activ- ity of protein C-I73N has been significantly impaired (Figure 7A and B). The lower activity of the protein C mutant is not due to its lower activation rate by thrombin- TM as demonstrated in the purified system (Figure 2F).
Discussion
We have demonstrated in this study that the I73N sub- stitution may be responsible for the recurrent DVT in the proband who is a compound heterozygote for I73N and R352Q mutations in PROC. In order to decipher the molecular basis of the anticoagulant defect, we expressed each mutant separately and characterized their properties in coagulation assays. We discovered the I73N substitu- tion introduces a potential new N-linked glycosylation site on EGF1-domain of protein C. This modification does not interfere with the activation of the mutant by thrombin- TM. However, the anticoagulant activity of APC-I73N was significantly decreased in both purified and plasma- based assays. Further analysis revealed APC-I73N exhibits
weaker affinity for protein S. This conclusion is derived from the observation that APC-I73N exhibited normal activity toward FVa in the absence of protein S, but impaired activity in the presence of the co-factor. In sup- port of this hypothesis, the anticoagulant activity of APC- I73N was normal in the protein S-deficient plasma, but reduced in the normal plasma. Furthermore, in thrombin generation assays, inhibitory activities of APC-I73N (nor- mal plasma) and protein C-I73N (protein C-deficient plas- ma supplemented with sTM) were markedly decreased. In contrast to I73N, the R352Q mutation did not impair the catalytic activity of APC in any of coagulation assays, strongly indicating the molecular defect leading to recur- rent DVT in the proband carrying the two mutations is primarily due to the I73N substitution. It should be noted that neither mutation adversely affected the anti-inflam- matory signaling function of APC.
Activated protein C is a Na+-binding protease and the R352Q mutation led to an approximately 2-fold lower affinity for interaction with the monovalent cation in both the absence and presence of Ca2+. We have previously demonstrated an allosteric linkage between the two metal
AB
C
D
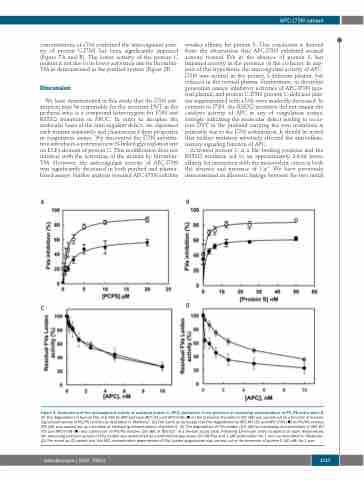
Figure 4. Assessment of the anticoagulant activity of activated protein C (APC) derivatives in the presence of increasing concentrations of PC/PS and protein S.
(A) The degradation of human FVa (2.5 nM) by APC-wild type (WT) () and APC-I73N () in the presence of protein S (20 nM) was carried out as a function of increas- ing concentrations of PC/PS vesicles as described in “Methods”. (B) The same as (A) except that FVa degradation by APC-WT () and APC-I73N () on PC/PS vesicles (25 μM) was carried out as a function of increasing concentrations of protein S. (C) The degradation of FVa Leiden (2.5 nM) by increasing concentrations of APC-WT () and APC-I73N () was carried out on PC/PS vesicles (25 μM) in TBS/Ca2+ in a 96-well assay plate. Following 10-minute (min) incubation at room temperature, the remaining co-factor activity of FVa Leiden was determined by a prothrombinase assay (10 nM FXa and 1 μM prothrombin for 1 min) as described in “Methods”. (D) The same as (C) except that the APC concentration dependence of FVa Leiden degradation was carried out in the presence of protein S (50 nM) for 1 min.
haematologica | 2020; 105(6)
1717