Page 260 - Haematologica - Vol. 105 n. 6 - June 2020
P. 260
Y. Lu et al.
ion binding loops, located in the C-terminal protease domain, modulates the catalytic activity of APC.6 We showed that binding of Ca2+ to 70-80-loop (chymotrypsin numbering)21 dramatically increases the affinity of Na+ for its specific site on 225-loop of APC. A similar Ca2+-depen- dent enhancement in Na+ affinity for APC-R352Q was observed (Kd(app) of 35mM and 2.8mM in the absence and presence of Ca2+, respectively). These dissociation con- stants are approximately 2-fold higher when compared to Na+ binding to APC-WT (Kd(app) of 18.7mM and 1.4mM in the absence and presence of Ca2+, respectively). These results suggest Arg352 contributes to high-affinity interac- tion of Na+ with 225-loop of APC, which has been deter- mined to be a monovalent cation-binding loop in all coag- ulation proteases.34 This residue is located near the S1 specificity site (Asp189) on an exposed surface loop (185- 189-loop) immediately below 225-loop.5 In the case of thrombin and factor Xa, mutagenesis studies have indicat- ed that residues of 185-189-loop are critical for catalytic activity and monovalent cation-binding specificity of these proteases.31-34 Thus, it appears that this loop makes a similar contribution to interaction of Na+ with APC. However, noting the high physiological concentration of Na+ in plasma and its high affinity for interaction with APC, a 2-fold lower affinity of Na+ for APC-R352Q has no physiological relevance. The normal activity of the mutant protein C/APC in the coagulation assays is consistent with this conclusion. Thus, the basis for the type-II protein C
deficiency observed with R352W mutation may be due to distortion of the active-site conformation of APC because of the large and hydrophobic nature of the Trp side- chain.35
The mechanism by which I73N mutation adversely affects the protein S-dependent anticoagulant function of APC is not fully understood. Similar to APC, protein S is a vitamin K-dependent protein which binds to negatively charged phospholipids. The affinity of protein S for phos- pholipid membranes is significantly higher than that of APC. It has been hypothesized that a co-factor function for protein S in the anticoagulant pathway is to stabilize the interaction of APC on phospholipid membranes in the vicinity of FVa/FVIIIa for optimal interaction and prote- olytic degradation of these procoagulant co-factors.9,12,28 Furthermore, there is some evidence to suggest an interac- tion with protein S is also associated with topographical changes in the active-site of APC, thereby aligning it with scissile bonds of co-factors on the membrane surface.13-15 Analysis of protein S and PC/PS concentration-depen- dence of FVa degradation by APC indicated an approxi- matley 2-fold weaker affinity for the mutant with protein S on PC/PS vesicles. While there was no difference in PC/PS concentration-dependence of FVa inactivation between APC-WT and APC-I73N in the absence of pro- tein S, the apparent dissociation constant for interaction with PC/PS in the presence of the co-factor was increased from 1 μM for WT to 2 μM for the mutant. These results
AB
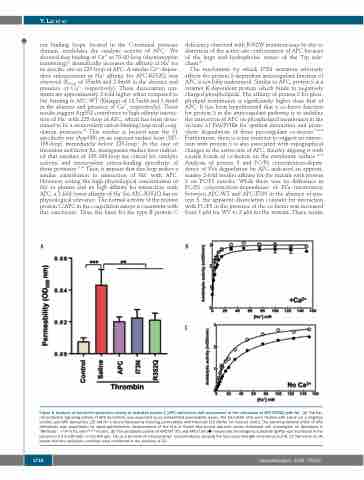
Figure 5. Analysis of the barrier-protective activity of activated protein C (APC) derivatives and assessment of the interaction of APC-R352Q with Na+. (A) The bar- rier-protective signaling activity of APC derivatives was evaluated by an established permeability assay. The EA.hy926 cells were treated with saline (as a negative control) and APC derivatives (25 nM for 3 hours) followed by inducing permeability with thrombin [10 nM for 10 minutes (min)]. The barrier-protective effect of APC derivatives was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional cell monolayers as described in “Methods”. **P<0.01 and P***<0.001. (B) The amidolytic activity of APC-WT () and APC-I73N () toward the chromogenic substrate SpPCa was monitored in the presence of 2.5 mM CaCl2 in Tris-HCl (pH, 7.4) as a function of increasing Na+ concentrations, keeping the total ionic strength constant at 0.2 M. (C) The same as (A) except that the amidolytic activities were monitored in the absence of Ca2+.
C
1718
haematologica | 2020; 105(6)