Page 262 - Haematologica - Vol. 105 n. 6 - June 2020
P. 262
Y. Lu et al.
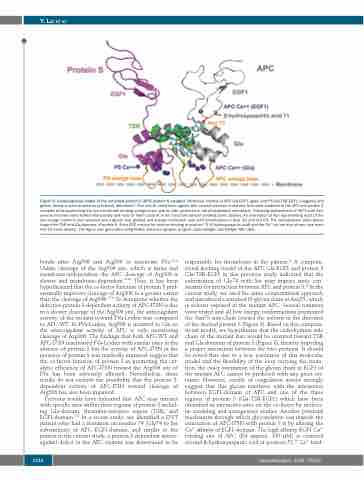
Figure 8. Computational model of the activated protein C (APC)-protein S complex. Molecular models of APC Gla-EGF1 (gray) and PS Gla-TSR-EGF1 (magenta and green) domains were docked as previously described.25 The overall orientation agrees with several previous mutations that were modeled in the APC and protein S complex while positioning the two membrane binding omega loops side by side anchored in the phospholipid membrane. Following replacement of Ile73 with Asn, several rotamers were tested interactively and most of them could fit in the structure without creating steric clashes. An orientation of Asn representing most of the low energy rotamers was selected and a glycan was grafted and energy minimized: cyan with heteroatoms in blue (N) and red (O). The carbohydrate chain points toward the TSR and Gla domains of protein S. A key APC residue for calcium binding at position 71 (β-hydroxyaspartic acid) and the Ca++ ion are also shown (see main text for more details). The figure was generated using PyMol molecular graphic program (Schrodinger, Cambridge, MA, USA).
bonds after Arg506 and Arg306 to inactivate FVa.36-38 Unlike cleavage of the Arg506 site, which is faster and membrane-independent, the APC cleavage of Arg306 is slower and membrane-dependent.36-38 Thus, it has been hypothesized that the co-factor function of protein S pref- erentially improves cleavage of Arg306 to a greater extent than the cleavage of Arg506.37,38 To determine whether the defective protein S-dependent activity of APC-I73N is due to a slower cleavage of the Arg306 site, the anticoagulant activity of the mutant toward FVa-Leiden was compared to APC-WT. In FVa-Leiden, Arg506 is mutated to Gln so the anticoagulant activity of APC is only monitoring cleavage of Arg306. The findings that both APC-WT and APC-I73N inactivated FVa-Leiden with similar rates in the absence of protein S but the activity of APC-I73N in the presence of protein S was markedly impaired, suggest that the co-factor function of protein S in promoting the cat- alytic efficiency of APC-I73N toward the Arg306 site of FVa has been adversely affected. Nevertheless, these results do not exclude the possibility that the protein S- dependent activity of APC-I73N toward cleavage of Arg506 has also been impaired.
Previous results have indicated that APC may interact with specific sites within three regions of protein S includ- ing Gla-domain, thrombin-sensitive region (TSR), and EGF1-domain.8-12 In a recent study, we identified a DVT patient who had a mutation on residue 74 (Gly74 to Ser substitution) of APC EGF1-domain, and similar to the patient in the current study, a protein S-dependent antico- agulant defect in the APC mutant was determined to be
responsible for thrombosis in the patient.25 A computa- tional docking model of the APC Gla-EGF1 and protein S Gla-TSR-EGF1 in this previous study indicated that the substitution of Gly74 with Ser may impose steric con- straints for interaction between APC and protein S.25 In the current study, we used the same computational approach and introduced a standard N-glycan chain at Asn73, which is solvent exposed in the mutant APC. Several rotamers were tested and all low energy conformations positioned the Asn73 side-chain toward the solvent in the direction of the docked protein S (Figure 8). Based on this computa- tional model, we hypothesize that the carbohydrate side chain of the mutant Asn would be oriented toward TSR and Gla domains of protein S (Figure 8), thereby impeding a proper interaction between the two proteins. It should be noted that due to a low resolution of this molecular model and the flexibility of the loop carrying the muta- tion, the exact orientation of the glycan chain in EGF1 of the mutant APC cannot be predicted with any great cer- tainty. However, results of coagulation assays strongly suggest that this glycan interferes with the interaction between EGF1-domain of APC and one of the three regions of protein S (Gla-TSR-EGF1) which have been identified as interactive-sites on the co-factor by molecu- lar modeling and mutagenesis studies. Another potential mechanism through which glycosylation can impede the interaction of APC-I73N with protein S is by altering the Ca2+ affinity of EGF1-domain. The high affinity EGF1 Ca2+ binding site of APC (Kd approx. 100 μM) is centered around β-hydroxyaspartic acid at position 71.39 Ca2+ bind-
1720
haematologica | 2020; 105(6)