Page 139 - Haematologica Vol. 107 - September 2022
P. 139
ARTICLE - A mouse model of humanized type 2B VWD
S. Kanaji et al.
analysis showed that hGPIba was shed off from the surface of the 2BEC mouse platelets (Figure 3C). The expression of hGPIba was preserved and platelets with spontaneously bound VWF was not abundantly present in 2BMK mice (Fig- ure 3C, upper right quadrant). These results demonstrate that severe thrombocytopenia and hGPIba cleavage ob- served with type 2B VWD model mice are primarily caused by mutant VWF synthesized in EC.
Enhanced megakaryocyte maturation in the bone marrow and extramedullary megakaryopoiesis in spleen Previous studies have indicated abnormal megakaryopoie- sis and thrombopoiesis in type 2B VWD. The challenging aspect of MK study using patients’ samples is the dif- ficulty in obtaining BM cells for analysis because BM as- pirate or biopsy is an invasive procedure especially for patients with a bleeding tendency. Therefore, earlier studies have been performed with MK differentiated in vitro from CD34+ cells isolated from patients’ peripheral
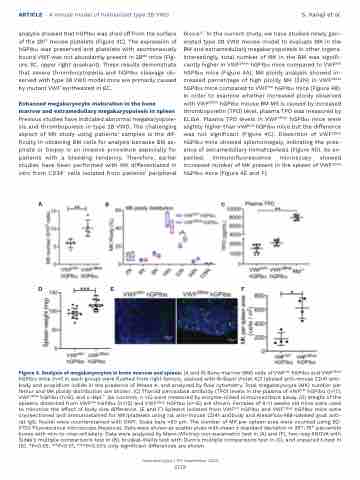
blood.6,7 In the current study, we have studied newly gen- erated type 2B VWD mouse model to evaluate MK in the BM and extramedullary megakaryopoiesis in other organs. Interestingly, total number of MK in the BM was signifi- cantly higher in VWF2Bhet hGPIba mice compared to VWFhA1 hGPIba mice (Figure 4A). MK ploidy analysis showed in- creased percentage of high ploidy MK (32N) in VWF2Bhet hGPIba mice compared to VWFhA1 hGPIba mice (Figure 4B). In order to examine whether increased ploidy observed with VWF2Bhet hGPIba mouse BM MK is caused by increased thrombopoietin (TPO) level, plasma TPO was measured by ELISA. Plasma TPO levels in VWF2Bhet hGPIba mice were slightly higher than VWFhA1 hGPIba mice but the difference was not significant (Figure 4C). Dissection of VWF2Bhet hGPIba mice showed splemomegaly, indicating the pres- ence of extramedullary hematopoiesis (Figure 4D). As ex- pected, immunofluorescence microscopy showed increased number of MK present in the spleen of VWF2Bhet hGPIba mice (Figure 4E and F).
ABC
DEF
Figure 4. Analysis of megakaryocytes in bone marrow and spleen. (A and B) Bone marrow (BM) cells of VWFhA1 hGPIba and VWF2Bhet hGPIba mice (n=5 in each group) were flushed from right femurs, stained with Brilliant Violet 421 labeled anti-mouse CD41 anti- body and propidium iodide in the presence of RNase A, and analyzed by flow cytometry. Total megakaryocyte (MK) number per femur and MK ploidy distribution are shown. (C) Thyroid peroxidase antibody (TPO) levels in the plasma of VWFhA1 hGPIba (n=7), VWF2Bhet hGPIba (n=6), and c-Mpl-/- (as controls, n =5) were measured by enzyme-linked immunosorbant assay. (D) Weight of the spleens dissected from VWFhA1 hGPIba (n=12) and VWF2Bhet hGPIba (n=15) are shown. Females of 9-11 weeks old mice were used to minimize the effect of body size difference. (E and F) Spleens isolated from VWFhA1 hGPIba and VWF2Bhet hGPIba mice were cryosectioned and immunostained for MK/platelets using rat anti-mouse CD41 antibody and AlexaFluor488-labeled goat anti- rat IgG. Nuclei were counterstained with DAPI. Scale bars =50 mm. The number of MK per spleen area were counted using BZ- X700 Fluorescence microscope (Keyence). Data were shown as scatter plots with mean ± standard deviation or 25th-75th percentile boxes with min-to-max-whiskers. Data were analyzed by Mann-Whitney non-parametric test in (A) and (F), two-way ANOVA with Šidák’s multiple comparisons test in (B), Kruskal-Wallis test with Dunn’s multiple comparisons test in (C), and unpaired t-test in (D). *P<0.05, **P<0.01, ***P<0.001; only significant differences are shown.
Haematologica | 107 September 2022
2138