Page 137 - Haematologica Vol. 107 - September 2022
P. 137
ARTICLE - A mouse model of humanized type 2B VWD
S. Kanaji et al.
thrombocytopenia is caused by gain-of-function VWF mutation only when species compatible GPIba is ex- pressed. Platelet size was increased in both VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice (Online Supplemen- tary Figure S1A). Decrease in the levels of VWF in plasma might be attributed to spontaneous binding to platelet GPIba and enhanced clearance of high molecular weight (HMW) VWF multimers. In support of this idea, VWF multimer analysis showed loss of HMW VWF multimers in the plasma of VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice, the effect was more profound in the latter (Figure 1D). In addition, increased susceptibility of type 2B mu- tant VWF to ADAMTS13 has been previously reported4,10 and this mechanism may also contribute to loss of HMW VWF multimers in our mice. Interestingly, plasma VWF level was also decreased in VWF2Bhomo mGPIba mice. In this strain, gain-of-function VWF has minimum sponta- neous binding to mGPIba expressed on platelet surface, and therefore, loss of HMW VWF multimer was less ob- vious compared to the strains with hGPIba. Decreased plasma VWF in this strain may be, at leaset partly, caused by impaired VWF synthesis, secretion or accel- erated degradation. Consistently, plasma VWF level of VWF2Bhomo hGPIba mice was lower than that of VWF2Bhet hGPIba mice despite similar severity of thrombocytope- nia, indicating the presence of additional mechanism for reduced VWF beyond spontaneous binding and clear-
ance. As expected, both VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice showed prolonged tail bleeding time which did not stop in 600 seconds (n=5 in each strain, Figure 1E).
Activation of circulating platelets in VWF2Bhet hGPIbα and VWF2Bhomo hGPIbα mice
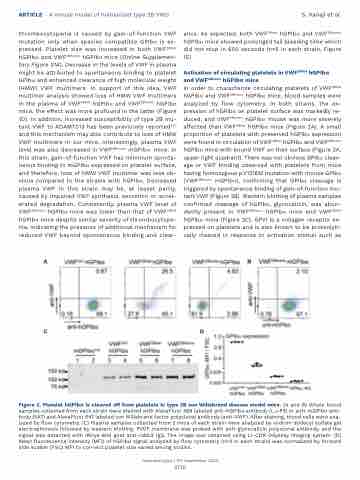
In order to characterize circulating platelets of VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice, blood samples were analyzed by flow cytometry. In both strains, the ex- pression of hGPIba on platelet surface was markedly re- duced, and VWF2Bhomo hGPIba mouse was more severely affected than VWF2Bhet hGPIba mice (Figure 2A). A small proportion of platelets with preserved hGPIba expression were found in circulation of VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice with bound VWF on their surface (Figure 2A, upper right quadrant). There was not obvious GPIba cleav- age or VWF binding observed with platelets from mice having homozygous p.V1316M mutation with mouse GPIba (VWF2Bhomo mGPIba), confirming that GPIba cleavage is triggered by spontaneous binding of gain-of-function mu- tant VWF (Figure 2B). Western blotting of plasma samples confirmed cleavage of hGPIba, glycocalicin, was abun- dantly present in VWF2Bhomo hGPIba mice and VWF2Bhet hGPIba mice (Figure 2C). GPVI is a collagen receptor ex- pressed on platelets and is also known to be proteolyti- cally cleaved in response to activation stimuli such as
AB
CD
Figure 2. Platelet hGPIbα is cleaved off from platelets in type 2B von Willebrand disease model mice. (A and B) Whole blood samples collected from each strain were stained with AlexaFluor 488 labeled anti-hGPIba antibody (LJ-P3) or anti-mGPIba anti- body (5A7) and AlexaFluor 647 labeled von Willebrand factor polyclonal antibody (anti-VWF). After staining, blood cells were ana- lyzed by flow cytometry. (C) Plasma samples collected from 2 mice of each strain were analyzed by sodium dodecyl sulfate gel electrophoresis followed by western blotting. PVDF membrane was probed with anti-glycocalicin polyclonal antibody, and the signal was detected with IRDye 800 goat anti-rabbit IgG. The image was obtained using LI-COR Odyssey imaging system. (D) Mean fluorescence intensity (MFI) of hGPIba signal analyzed by flow cytometry (n=4 in each strain) was normalized by forward side scatter (FSC) MFI to cor-rect platelet size varied among strains.
Haematologica | 107 September 2022
2136