Page 136 - Haematologica Vol. 107 - September 2022
P. 136
ARTICLE - A mouse model of humanized type 2B VWD
S. Kanaji et al.
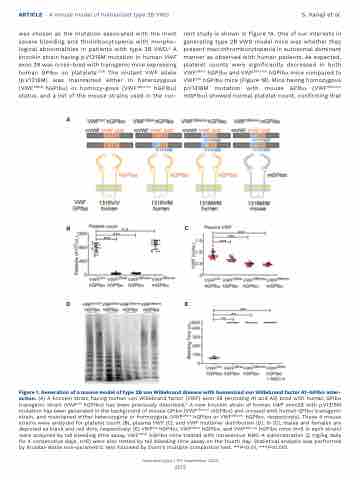
was chosen as the mutation associated with the most severe bleeding and thrombocytopenia with morpho- logical abnormalities in patients with type 2B VWD.5 A knockin strain having p.V1316M mutation in human VWF exon 28 was cross-bred with transgenic mice expressing human GPIba on platelets.13,18 The mutant VWF allele (p.V1316M) was maintained either in heterozygous (VWF2Bhet hGPIba) or homozy-gous (VWF2Bhomo hGPIba) status, and a list of the mouse strains used in the cur-
rent study is shown in Figure 1A. One of our interests in generating type 2B VWD model mice was whether they present macrothrombocytopenia in autosomal dominant manner as observed with human patients. As expected, platelet counts were significantly decreased in both VWF2Bhet hGPIba and VWF2Bhomo hGPIba mice compared to VWFhA1 hGPIba mice (Figure 1B). Mice having homozygous p.V1316M mutation with mouse GPIba (VWF2Bhomo mGPIba) showed normal platelet count, confirming that
A
BC
DE
Figure 1. Generation of a mouse model of type 2B von Willebrand disease with humanized von Willebrand factor A1-GPIbα inter- action. (A) A knockin strain having human von Willebrand factor (VWF) exon 28 (encoding A1 and A2) bred with human GPIba transgenic strain (VWFhA1 hGPIba) has been previously described.13 A new knockin strain of human VWF exon28 with p.V1316M mutation has been generated in the background of mouse GPIba (VWF2Bhomo mGPIba) and crossed with human GPIba transgenic strain, and maintained either heterozygote or homozygote (VWF2Bhet hGPIba or VWF2Bhomo hGPIba, respectively). These 4 mouse strains were analyzed for platelet count (B), plasma VWF (C), and VWF multimer distribution (D). In (C), males and females are depicted as black and red dots, respectively. (E) VWFhA1 hGPIba, VWF2Bhet hGPIba, and VWF2Bhomo hGPIba mice (n=5 in each strain) were analyzed by tail bleeding time assay. VWF2Bhet hGPIba mice treated with intravenous NMC-4 administration (2 mg/kg daily for 4 consecutive days, n=6) were also tested by tail bleeding time assay on the fourth day. Statistical analysis was performed by Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparison test. **P<0.01, ***P<0.001.
Haematologica | 107 September 2022
2135