Page 272 - 2022_01-Haematologica-web
P. 272
M. Faleschini et al.
tion of a cryptic acceptor splicing site (ggagtgtcctgttc- cgcaggggat) with a score of 0.83 predicted by NNSpice tool (Figure 2B). Therefore, the two additional products would correspond to GFI1B molecules of an expected molecular weight of 39 kDa (p39) and 34 kDa (p34), respectively for an in-frame insertion of 22 aa (p.216_217insGIPAGSSPEPAPDPPGPHFLRQ).
We investigated the expression level of the four forms of GFI1B (Online Supplementary Table S2). In control samples (n=2), forms p37 and p32 represented the most expressed products, being approximately 83% and 10% of the total GFI1B cDNA, respectively; the other two forms, p34 and p39, were overlooked (Figure 2C). In patients, the expres- sion level of p37 and p32 was significantly different than in controls, corresponding to 54% and 32% of the total cDNA, respectively. Of note, p32 was relatively less expressed than p37, suggesting that c.648+5G>A leads to partial skipping of exon 9 or partial degradation of the mRNA. Finally, no significant difference in the expression of p39 and p34 was observed between patient and control samples.
Pathogenic role of the c.648+5G>A mutation leading to p32
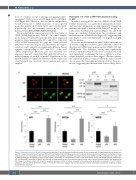
In order to investigate the effect of c.648+5G>A on GFI1B cellular localization, we performed immunofluorescence and western blot analysis in cells transiently transfected with the wild-type or mutant cDNA. Like p37, the p32 iso- form enters the HeLa cell nucleus (Figure 3A) and both forms are similarly distributed in the cytoplasmic and nuclear fractions of Hek293 cells (Figure 3B), suggesting that zinc fingers 1 and 2 are dispensable for migration of p32 into the nucleus.
Moreover, we determined the effect on the transcription- al activity, using the luciferase gene under the control of three known GFI1B target gene promoters (MEIS, GFI, and GFI1B itself). The luciferase activity was significantly reduced when the Meg-01 cells were co-transfected with myc-tagged p37, confirming the role of GFI1B as a tran- scription repressor of those three targets.27 On the contrary, the expression of p32 not only abolished the repression but also increased the transcriptional activity of those three pro- moters, suggesting that the functional defect is likely due to
AB
C
Figure 3. Pathogenic role of GFI1B p32. (A) Immunofluorescence (IF) and (B) western blot (WB) of nuclear (N) and cytoplasmatic (C) fractionated cellular lysates from Hek293 transiently transfected with wild-type (WT) (p37) and mutated (p32) GFI1B expression vector. Results demonstrate that both isoforms are distributed in the nucleus as well as in the cytoplasm with the same proportions. In IF, propidium iodide (PI) was used to detect nuclei. HSP90 and ORC2 antibodies were used in WB as loading marker for the cytoplasmic and nuclear fraction, respectively. (C) Transcriptional activity of p37 (WT) and the variant p32 GFI1B isoforms measured on GFI, GFI1B and MEIS promoters in Meg01 cells. Results are represented by ratio of the promoter signal (Firefly) and the control promoter (Renilla) and expressed as fold change in luciferase activity. p37 shows the expected repressive activity on targets compared to the empty vector while p32 is an activator (1- to >1.5-fold) in comparison with the p37 for all the three targets (***P<0.001). Error bars represent the standard deviation of three independent experiments. Statistical analysis was performed using t-test.
264
haematologica | 2022; 107(1)