Page 160 - 2021_12-Haematologica-web
P. 160
M.N. Zeissig et al.
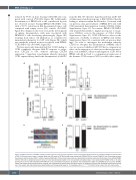
reduced by 97.8% in mice bearing CCR1 KO cells com- pared with controls (P<0.0001; Figure 5B). Additionally, dissemination of OPM2 cells to the contralateral leg was not observed in mice bearing OPM2-CCR1-KO-1 cells, with a 99.9% reduction in BM disseminated tumor cells compared with empty vector (EV) controls (P<0.0001; Figure 5C). Similar results were seen in the development of splenic dissemination, with mice inoculated with OPM2-EV-1 cells developing splenomegaly (Figure 5D) resulting from tumor cell infiltration, as confirmed by immunohistochemistry for GFP+ cells (Figure 5E), which was markedly reduced in mice inoculated with OPM2- CCR1-KO-1 cells (P<0.0001; Figure 5D).
We have previously demonstrated that CCL3 binding to CCR1 completely abrogates MM PC response to exoge- nous CXCL12 in vitro, without affecting CXCR4 expression,17 suggesting a mechanism whereby increased CCR1 expression may enable the dissemination of MM PC
AB
from the BM. We therefore hypothesized that CCR1 KO cell lines may retain their response to BM CXCL12, thereby leading to retention within the BM niche. Consistent with our previous data, pre-treatment of OPM2-EV-1 cells with CCL3 prevented their migration towards CXCL12 (Online Supplementary Figure S2). In contrast, OPM2-CCR1-KO-1 cells retained their ability to migrate in response to exoge- nous CXCL12, even in the presence of CCL3 (Online Supplementary Figure S2). CCR1 KO had no effect on the expression of CXCR4 or CXCL12 in OPM2 cells (Online Supplementary Figure S3), consistent with our previous find- ings.17 In order to investigate the mechanism whereby CCR1 loss abrogates the dissemination of OPM2 cells in vivo, we assessed whether CCR1 KO had a compensatory effect on the expression of other factors that are known to play a role in MM PC adhesion and migration. CCR1 KO in OPM2 cells did not lead to a compensatory expression of the alternate CCL3 receptor CCR5, nor did it affect expres-
CD
EF
Figure 3. CCR1 expression in 5TGM1 multiple myeloma plasma cells increases incidence of bone and splenic dissemination in a C57BL/KaLwRij intratibial model of MM. (A) Primary tumor burden in injected tibiae after 3.5 weeks in C57BL/KaLwRij mice injected with 5TGM1-CCR1 or control 5TGM1-EV cells. Percentage of green fluorescence protein positive (GFP+) multiple myeloma (MM) cells of total mononuclear cells were quantitated using flow cytometry. (B) Number of circulating 5TGM1- CCR1 or -EV cells in peripheral blood of mice. (C) Tumor burden disseminated to the non-injected contralateral leg in mice injected with 5TGM1-CCR1 or -EV cells. (D) Proportion of mice with detectable GFP+ MM cells in the contralateral long bones. (E) Spleens were collected from eight mice (5TGM1-EV) and 11 mice (5TGM1- CCR1) and imaged using bioluminescence imaging, with representative spleens from each group shown. (F) Proportion of mice with detectable bioluminescence sig- nal in the spleen. Box and whisker plots depict median and interquartile range for 11 mice/group (A to C). ****P<0.0001, Fisher’s exact test. EV: empty vector.
3180
haematologica | 2021; 106(12)