Page 90 - 2021_07-Haematologica-web
P. 90
K. Klausz et al.
AB
C
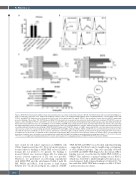
Figure 2. MSH-TP15 binds human ICAM-1 domain 1-2 and partly shares an epitope with CD54 antibodies BI-505 and 84H10. (A) In order to proof that binding speci- ficity is retained by conversion of the single-chain fragment variable (scFv) to the antigen-binding fragment (Fab) containing antibody, cross-blocking studies with TP15-Fc and MSH-TP15 antibodies were performed on L363 cells. Pre-incubation with 500 mg/mL TP15-Fc, but not with the control molecule 4D5-Fc, significantly prevented binding of Alexa Fluor 755 (AF755)-labeled MSH-TP15 (checked bar) measured by flow cytometry. Identically labeled cetuximab (CTX-AF755) served as negative control. Binding of TP15-Fc and 4D5-Fc were detected with FITC-labeled anti-human immunoglobulin (Ig) G antibody (grey bars). (B) Antibody binding to human intercellular adhesion molecule-1 (huICAM-1) or mouse ICAM-1 (muICAM-1) was analyzed by flow cytometry. Transiently transfected CHO-K1 cells expressing myc-tagged full length or truncated human ICAM-1 domains (D) D1-2 to D1-5 were stained with mouse anti-myc antibody and detected with a FITC-labeled anti-mouse secondary antibody. Histograms show binding of MSH-TP15-AF755 (grey) and CTX-AF755 (black) on ICAM-1 expressing CHO-K1 cells. Non-transfected (non-transf.) cells served as control. (C) Cross-blocking experiments with MSH-TP15 and ICAM-1 antibodies RR1/1, 84H10 and BI-505 were performed by flow cytometry on L363 cells with directly labeled antibodies or species specific anti-human or anti-mouse IgG secondary antibody as indicated in the graphs. All graphs show mean values ± standard error of the mean of a minimum of three independent experiments. MFI: mean fluorescence intensity. Controls are shown in grey, cross-blocking in pat- terned bars. Statistics were calculated vs. pre-incubation with control IgG1 (ctrl IgG1) or binding of antibody without blocking (black bars). ***P<0.001, **P<0.01, *P<0.05, n.s: not significant. Drawing summarizes the results and visualizes the overlapping epitopes of MSH-TP15, BI-505 and clone 84H10.
were cloned for cell surface expression on CHO-K1 cells (Online Supplementary Figure S1). Flow cytometric analyses revealed that for binding of MSH-TP15 at least ICAM-1 domain (D) 1-2 needed to be present. No binding to mouse ICAM-1 was observed (Figure 2B). For unknown reasons isolated expression of D1 was not possible. Therefore, we performed cross-blocking experiments with MSH-TP15 and the anti-human ICAM-1 mAb BI- 505, 84H10 and RR1/1, both known to bind human ICAM-1 D1.30 As shown in Figure 2C, addition of MSH-
TP15, BI-505 and 84H10 cross-blocked individual binding – suggesting that they bound to neighboring, overlapping, or even identical epitopes. This was especially observed for BI-505 and MSH-TP15 (Figure 2C). In contrast, RR1/1 binding was not cross-blocked by MSH-TP15, BI-505 or 84H10. This indicates a unique binding site for RR1/1, which was described to inhibit lymphocyte function-asso- ciated antigen-1 (LFA-1) ligand binding to ICAM-1 D1.31 In line with this, MSH-TP15 had no impact on LFA-1-ICAM- 1 interaction (data not shown).
1860
haematologica | 2021; 106(7)