Page 205 - 2021_07-Haematologica-web
P. 205
Pim kinase regulates TP receptor signaling
A (i)
(ii) B (i) (ii)
C (i) (ii)
D (i)
(iii)
(ii)
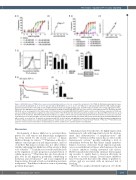
Figure 6. AZD1208 reduces TPaR surface expression and signaling and does not act as a competitive antagonist of the TPaR. (A, B) Human washed platelets were pre-treated with: (A) AZD1208 (1, 3, 10, 30, 50 and 100 mM) or (B) GR32191 (1, 2, 3, 5, 10, 30, 100 nM) prior to stimulation with U46619 (3 nM–3 mM) and aggre- gation was monitored after 5 min using an optical light transmission plate-based aggregometry assay. Quantified data are shown. (i) Percentage aggregation. (ii) EC50 values from the aggregation dose-response curves determined following incubation with AZD1208 were used to plot a Schild regression plot to determine whether AZD1208 acts as an antagonist for the TxA2 receptor. (C) Platelets were treated with AZD1208 (10 mM; 10 mins) or vehicle control. Surface expression of TPaR was assessed using an antibody that recognizes the extracellular portion of the TPaR and detected by flow cytometry. Samples were diluted in HBS and not fixed to avoid disruption of the membrane. Anti-DOK6 antibody was included as a negative control. (i) A representative histogram from the flow cytometer. (ii) Data are expressed as median fluorescent intensity (MFI). (iii) Total cellular TPaR was detected by western blotting. (D) Human platelet-rich plasma was pretreated with AZD1208 (100 mM) or vehicle, as a control, for 10 min prior to stimulation with SDF-1a (200 ng/mL) and aggregation was monitored using optical light transmission aggregometry for 5 min. (i) Representative trace and (ii) quantified data are shown. Results are mean + standard error of mean for n≥3, *P≤0.05, **P≤0.01, ***P≤0.005 in com- parison to vehicle-treated control; where normalized data are shown, statistics were performed prior to normalization.
Discussion
Development of kinase inhibitors as potential thera- peutics for solid tumors and hematologic malignancies has been fueled by the recent successes of kinase inhibitor therapy for cancers.1,2,31,32 Pim kinase is known to enhance cancer progression and drug resistance, and loss of all three Pim kinase isoforms does not affect embryo viability, indicating that inhibition of Pim activity is likely to be tolerable.10,11,33 In addition to its well-established role in the regulation of cycle progression and prevention of cellular apoptosis,3-6,23 Pim kinase has also been shown to play roles in cell migration, potentially contributing to metastasis and cell invasion18,19 and is also implicated in drug resistance through activation of multidrug resistance transporters.7-9 Pim kinase is therefore seen as a promising potential drug target.
Pim kinases have been shown to be highly expressed in hematopoietic cells with important roles in the develop- ment and differentiation of megakaryocytes10 and platelets.11,15 Deletion of Pim-1 alone has no effect on the hematopoietic system,16 possibly indicating a level of redundancy between the Pim kinase family members. Kinases, however, often have broad expression profiles across several different cell types, which increases the risk of kinase inhibitors having unwanted side effects. Platelets in particular rely heavily on kinase-driven signal- ing cascades to enable them to function effectively in response to vascular damage. Several kinase inhibitors have been reported that affect the ability of platelets to activate and are associated with an increased risk of bleeding.34-36
Western blot analysis identified expression of both the
haematologica | 2021; 106(7)
1975