Page 204 - 2021_07-Haematologica-web
P. 204
A.J. Unsworth et al.
A (i)
(ii)
B (i)
(ii) C (i) (ii)
D (i)
(ii) E (i) (ii)
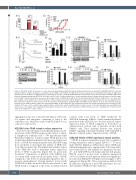
Figure 5. AZD1208 inhibits thromboxane receptor signaling. (A) Resting and stimulated human washed platelets were treated with 10 mM AZD1208 for 10 min and stimulated with (i) collagen (1 mg/mL) or arachidonic acid (1 mM) or (ii) U46619 (0-3 mM) in the presence and absence of indomethacin (10 mM). (i) TxB2 levels (EnzoLife Sciences ELISA) or (ii) aggregation were monitored after 5 min of shaking. (B) Mobilization of intracellular calcium was determined in FURA-2 AM loaded platelets following stimulation with U46619 (300 nM) or ADP (10 mM). (i) Representative traces and (ii) quantified data are shown, with data expressed as the change in [Ca2+] (nM). (C-E) Human platelets were pre-incubated with vehicle or AZD1208 (10 mM) for 10 min and stimulated with U46619 (1 mM) for 30 s or 3 min before lysis in SDS Laemmli sample buffer. (C) Protein kinase C (PKC) activity was determined by blotting these samples and using a phospho-site specific antibody (for the PKC substrate recognition sequence) that detects PKC substrate phosphorylation. (D) Myosin light chain (MLC) phosphorylation at Ser19 was determined using a phospho-specific antibody that recognizes the phosphorylated MLC. (E) Phosphorylation of the integrin β3 subunit at Y773 was determined using a phospho-specific antibody. Actin was used to confirm equal loading. (i) Representative blots and (ii) quantified data are shown. Levels of total phosphorylation were quantified and expressed as a percentage of the maximum phosphorylation observed in vehicle-treated, stimulated controls. Results are mean + standard error of mean for n≥3, *P≤0.05 in comparison to vehicle controls.
aggregation assay was consistent with that of a TP recep- tor agonist and antagonist competing to bind to the orthosteric site, while the results with AZD1208 do not conform to this model.
AZD1208 alters TPaR receptor surface expression
It has been described previously that Pim kinase modu- lates levels of the CXCR4 receptor at the surface of chron- ic lymphocytic leukemia cells.18,19 We hypothesized that Pim kinase inhibitors could modulate TPaR function via a similar mechanism in platelets and measured expression levels of TPaR following treatment with AZD1208 using flow cytometry to investigate this further. A TPaR anti- body that recognizes the N-terminal (extracellular) region of the TPaR was used to determine surface expression levels of TPaR on platelets (Figure 6C). As previously described,27 stimulation of platelets with U46619 was associated with a reduction in cell surface levels of TPaR, compared to the levels on unstimulated platelets, due to receptor internalization. Resting platelets treated with AZD1208 (10 mM) showed reduced surface levels of the TPaR receptor compared to the levels in vehicle-treated
controls with total levels of TPaR unaffected by AZD1208 treatment. U46619- (1 mM) stimulated platelets pretreated with AZD1208 also showed reduced surface expression levels of TPaR compared to both those of vehicle-treated controls and of platelets treated with U46619 only. These findings indicate that reduced U46619 signaling following treatment with AZD1208 is linked to reduced surface expression levels of TPaR.
AZD1208 inhibits CXCR4 signaling in human platelets
CXCR4 is expressed in platelets and is able to signal fol- lowing stimulation with its ligand SDF-1a.28-30 To deter- mine whether inhibition of Pim kinase alters CXCR4 sig- naling in human platelets, aggregometry following stimu- lation with SDF-1a (200 ng/mL) was performed in vehi- cle- and AZD1208-treated human platelet-rich plasma. SDF-1a caused a modest level of platelet aggregation (~50%) in vehicle-treated platelets which was significant- ly reduced in AZD1208- (100 mM) treated samples (~20% aggregation) (Figure 6D). These observations indicate that in addition to regulation of the TPaR, Pim kinase also reg- ulates platelet CXCR4 receptor function.
1974
haematologica | 2021; 106(7)