Page 203 - 2021_07-Haematologica-web
P. 203
Pim kinase regulates TP receptor signaling
A B (i) (ii)
C (i) C (ii) D
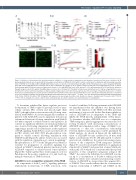
Figure 4. Inhibition of thromboxane A2 signaling underlies inhibition of collagen-induced aggregation and thrombus formation by Pim kinase inhibitors. (A, B) Human washed platelets were treated with (A) increasing concentrations of AZD1208 (1-100 mM) or vehicle control prior to stimulation with increasing concentra- tions of U46619 (0.03-10 mM) or (B) 10 mM AZD1208 in the presence or absence of (i) indomethacin (10 μM) or (ii) the thromboxane A2 receptor antagonist GR32191 (100 nM). Platelet aggregation was monitored after 5 min of stimulation by collagen (0.01-10 mg/mL) using a 96-well plate based aggregometry assay. (C) DiOC6 loaded human whole blood was pretreated with vehicle (black) or 100 mM AZD1208 (red), in the presence of 10 mM indomethacin for 10 min before perfusion through collagen coated (100 mg/mL) Vena8Biochips at a shear rate of 20 dyn/cm2. Thrombus formation was determined over 10 min by comparing fluorescence intensity in the vehicle and treated samples. (i) Representative images taken at 10 min. (ii) Data expressed as the percentage of maximum fluorescence of vehicle treated cases and normalized to an untreated (no indomethacin treatment) control, where the maximum fluorescence observed in untreated platelets is considered to be 100% thrombus formation. (D) Fibrinogen binding in washed platelets from control (Pim1+/-) or Pim1-/- mice was determined following stimulation with thrombin (0.01 U/mL), U46619 (10 mM) or CRP (10 mg/mL) and expressed as percentage of positive cells. Results are mean + standard error of mean for n≥3, *P≤0.05 ***P≤0.005 in comparison to vehicle control; where normalized data are shown, statistics were performed prior to normalization.
To determine whether Pim kinase regulates processes downstream of TPaR coupled G proteins, levels of intra- cellular calcium, PKC activity and myosin light chain (MLC) phosphorylation were monitored following stim- ulation with U46619. As shown in figure 5B, treatment of platelets with AZD1208 caused a significant reduction in calcium mobilization following stimulation with U46619 (0.3 mM) compared to vehicle-treated control platelets. In contrast no significant difference in calcium mobilization was observed following stimulation with ADP (10 mM) supporting a specific role for Pim kinase in the regulation of TPaR signaling. AZD1208 also caused a reduction both in PKC activity and MLC (S19) phosphorylation com- pared to vehicle controls following stimulation with U46619 (1 mM) (Figure 5C and D). Ga13 is also associated
found to be inhibited following treatment with AZD1208 we hypothesized that the inhibitor was having direct effect on the function of the TPaR itself. One potential mechanism of action could be that Pim kinase inhibitors such as AZD1208 act as antagonists of the TPaR and inhibit the TPaR directly, independently of Pim kinase. To determine whether AZD1208 acts as a competitive TPaR antagonist, platelet aggregation was measured fol- lowing treatment with increasing concentrations of AZD1208 and stimulation with a range of U46619 con- centrations so that the concentration relationship between inhibitor and antagonist could be quantified by Schild analysis. As shown in Figure 6A the inhibitory effect of AZD1208 became saturated by 10 mM, with higher concentrations (30, 50, 100 mM) unable to achieve greater levels of inhibition. In contrast, increasing concen- trations of the competitive TPaR antagonist GR32191 (Figure 5) caused non-saturable inhibition of aggregation stimulated by U46619 and generated a linear Schild plot with a pA2 of 8.9 which was consistent with the reported properties of this competitive antagonist acting at the TPaR.26 In contrast, the Schild plot for AZD1208 was lin- ear up to a concentration of 10 mM at which point the inhibitory effects were saturated and increasing concen- trations of AZD1208 no longer altered the apparent EC of U46619. These results indicate that the concentration- response relationship of GR32191 and U46619 in the
with the regulation of integrin a β IIb 3
outside in signaling.25 Phosphorylation of Y773 on the integrin β3 tail which is essential for propagation of outside-in signaling was reduced in U46619 stimulated platelets pretreated with AZD1208 compared to vehicle-treated control platelets (Figure 5E). Taken together these results support a role for Pim kinase in the positive regulation of G and Ga13 sig-
naling downstream of TPaR activation. q
AZD1208 is not a competitive antagonist of the TPaR
50
As components of both G and G signaling, pathways q 13
immediately downstream of TPaR activation, were
haematologica | 2021; 106(7)
1973