Page 201 - 2021_07-Haematologica-web
P. 201
Pim kinase regulates TP receptor signaling
A (i) (ii)
B
C (i) (ii)
D (i) (ii)
E (i)
F (i)
(ii)
F (ii) G
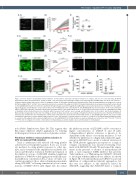
Figure 2. Genetic deletion and pharmacological inhibition of Pim-1 kinase attenuates thrombus formation on collagen, but does not cause bleeding. (A) DiOC6 loaded mouse whole blood from Pim1+/- (black) or Pim1-/- (red) mice was perfused through collagen-coated (100 mg/mL) Vena8Biochips at a shear rate of 1500 s-1. (i) Representative images taken at the end of recording are shown. (ii) Thrombus formation was determined after 4 min by comparing the percentage area covered. (B) Tail bleeding in Pim1+/- or Pim1-/- mice represented as time to cessation of bleeding (s). (C-E) DiOC6 loaded human whole blood was pretreated with vehicle (black) or 100 mM AZD1208 (red) for 10 min before perfusion through collagen-coated (100 mg/mL) Vena8Biochips at (C) an arterial shear rate of 20 dyn/cm2, (D) a patho- logical shear rate of 135 dyn/cm2, or (E) a venous shear rate of 4.5 dyn/cm2. The concentration of 100 mM AZD1208 was chosen because of the reduced bioavail- ability of AZD1208 in plasma. Thrombus formation was determined after 5 min (pathological shear) or 10 min (arterial and venous shear) by comparing percentage of maximum vehicle-treated fluorescence intensity, which measures both surface area coverage and thrombus size, in the vehicle and treated samples. (i) Representative images taken at the end of recording are shown. (ii) Data expressed as percentage of maximum vehicle-treated fluorescence intensity. (F) Thrombus formation was determined in vivo following ferric chloride-induced injury in mice pretreated for 10 mins with vehicle or 100 μM AZD1208. DiOC6 was used to enable visualization of platelets. (i) representative images taken at 0, 300, 600 and 900 s. (ii) Data expressed as time to occlusion (s). (G) Tail bleeding determined as time to cessation of bleeding (s) in mice pretreated with vehicle or 100 mM AZD1208 for 10 min. Results are mean ± standard error of mean for n≥3, *P≤0.05, **P≤0.01 ****P≤0.001 in comparison to vehicle control; where normalized data are shown statistics were performed prior to normalization.
trols (Online Supplementary Figure S6). This suggests that Pim kinase inhibitors inhibit aggregation by reducing both integrin activation and secretion of granule contents.
Pim kinase inhibitors reduce platelet activation to GPVI via reduced TPaR signaling
TxA2 is synthesized and released following platelet activation by several platelet agonists. It acts as a second- ary mediator, boosting platelet responses to other ago- nists, and is essential for the amplification of platelet acti- vation and thrombus formation. Further investigation of the inhibitory actions of AZD1208 identified significant inhibition of U46619-evoked aggregation with half maxi- mal inhibitory concentration (IC50) values <10 mM, con- centrations similar to those achieved in plasma in patients taking AZD1208,24 following stimulation by low concen- trations of U46619 (0.03-1 mM) (Figure 4A). In contrast
the inhibitory activity of AZD1208 could be overcome at higher concentrations of U46619 (3 and 10 mM). Collagen-induced platelet activation is known to be dependent on the release of secondary mediators. To test whether the inhibition of collagen-induced platelet aggre- gation by AZD1208 was a result of reduced activation of the TPaR, platelet aggregation in response to a range of collagen concentrations was measured following treat- ment with AZD1208 (10 mM). We performed the experi- ments in the presence or absence of indomethacin (10 mM), a cyclo-oxygenase (COX) inhibitor that prevents TxA2 synthesis, or a TPaR antagonist GR32191 (100 nM) to investigate whether inhibition was additive. As shown in Figure 4B, both indomethacin and GR32191 caused an inhibition of collagen-induced platelet aggregation, but this was not additive to the inhibition caused by AZD1208, suggesting that AZD1208 shares a common
haematologica | 2021; 106(7)
1971