Page 147 - 2021_07-Haematologica-web
P. 147
Hematopoietic JAK 2V617F in aortic aneurysms
the Ang II-infused JAK2V617F-BMT mice (Figure 7B; Online Supplementary Figure 7A), but did not affect the levels of blood pressures or heart rate (Figure 7C; Online Supplementary Figure S7B to D). Total cholesterol and triglyceride concentrations were increased in the ruxoli- tinib-treated JAK2V617F-BMT mice compared to the vehi- cle-treated JAK2V617F-BMT mice, as previously reported in MPN patients (Online Supplementary Table S5). Importantly, abdominal aorta diameters were significant- ly decreased in the ruxolitinib-treated JAK2V617F-BMT mice compared to the vehicle-treated JAK2V617F-BMT mice at both 2 and 4 weeks after Ang II infusion (Figure 7D and 7E). The incidence of AAA was 64% of the vehi- cle-treated JAK2V617F-BMT mice (7 of 11) while 18% of the ruxolitinib-treated JAK2V617F-BMT mice (2 of 11). Accordingly, the Kaplan-Meier analysis demonstrated that the AAA-free survival rate was significantly higher in the ruxolitinib-treated JAK2V617F-BMT mice than in the vehicle-treated JAK2V617F-BMT mice (Figure 7F). Ruxolitinib decreased MMP-2 activity and pro MMP-9 expression in the abdominal aorta in Ang II-infused JAK2V617F-BMT mice (Figure 7G). In contrast, thoracic aorta diameters or incidence of TAA were not different between the ruxolitinib- and vehicle-treated JAK2V617F- BMT mice (Online Supplementary Figure S7E and S7F). Taken together, these findings strongly suggest that hematopoietic JAK2 V617F plays a causal role in the onset and development of AAA.
A
Discussion
The present study is the first to demonstrate that hematopoietic cells carrying JAK2 V617F are causally associated with AAA formation in mice. In response to Ang II stimulation, the infiltrated CD68+ macrophages with JAK2 V617F in the arterial walls may have con- tributed to the genesis and activation of matrix metallo- proteinase, resulting in increases of elastin degradation in the abdominal aorta and the development of AAA. Our findings provide a novel feature of vascular complication of AA in MPN patients due to the constitutive activation of the hematopoietic JAK-STAT pathway.
To date, vascular complications of MPN patients have been reported mainly in the arterial and venous throm- botic events from clinical and murine studies.3,39 History of thrombosis and age >60 years are the highest risk fac- tors for thrombosis in MPN patients.40 Interestingly, JAK2 V617F-positive MPN patients with a history of thrombo- sis more frequently showed AA, suggesting that history of thrombosis may be a risk factor for AA as well as thrombosis, whereas age was not different between JAK2 V617F-positive MPN patients with AA and those without AA. Leukocytosis is another factor that increases the risks of thrombosis in MPN patients whereas the roles of increased number or functional alteration of platelets have been controversial in cases of thrombosis in MPN,3,41 indicating that abnormal MPN-clone-derived leukocytes
B
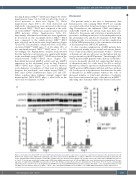
Figure 4. Hematopoietic JAK2 V617F increases STAT3 phosphorylation and cytokine expression in response to angiotensin II infusion in the abdominal aorta. (A) Western blot analysis on the STAT3 in the abdominal aorta. Aorta extracts from the WT-BMT mice or JAK2V617F-BMT mice were immunoblotted with the indicated anti- bodies. Phosphorylated STAT3 (p-STAT3) to total STAT3 (t-STAT3) ratios are shown in the bar graphs. The average value for the saline-infused WT-BMT mice was set to 1 (n=5–6). β actin was used as the loading control. (B) Relative mRNA expression levels of Ccl6 and Tgfb1 in the aorta. Actb was used for normalization. The aver- age value for saline-infused WT-BMT mice was set to 1 (n=7–10). All data are presented as mean ± standard error of the mean. *P<0.05 vs. the corresponding saline-infused mice and †P<0.05 vs. the Ang II-infused WT-BMT mice by one-way ANOVA with Tukey post-hoc analysis. WT, ApoE−/−: mice transplanted with bone mar- row (BM) cells from wild-type (WT) mice; JAK2V617F, ApoE−/−: mice transplanted with BM cells from JAK2V617F mice; BMT: BM transplantation; Ang II: angiotensin II.
haematologica | 2021; 106(7)
1917