Page 148 - 2021_07-Haematologica-web
P. 148
T. Yokokawa et al.
A
B
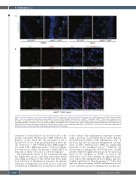
Figure 5. Characterization of bone marrow-derived JAK2 V617F hematopoietic cells in the abdominal aorta by use of GFP-transgene. (A) The lethally irradiated ApoE−/− mice were transplanted with bone marrow (BM) cells from the WT/CAG-EGFP (WT-GFP) mice or JAK2V617F/CAG-EGFP (JAK2V617F-GFP) double transgenic mice. The ApoE−/− recipient mice were subjected to saline or angiotensin II (Ang II) infusion for 4 weeks. The abdominal aortas were stained with an anti-GFP (green) anti- body and DAPI (blue). Scale bars, 100 mm. (B) Representative immunofluorescence images of the aorta sections stained with anti-CD45 (red), anti-CD68 (red), or anti-Ly6B.2 (red) and anti-GFP (green) antibodies and DAPI (blue) in ApoE−/− mice transplanted with JAK2V617F-GFP BM cells. Scale bars, 50 mm. Ang II: angiotensin II; GFP: green fluorescent protein; DAPI: 4′,6-diamidino-2-phenylindole; WT: wild-type.
contribute to vascular diseases. In our cohort, 23% of the patients with JAK2 V617F-positive MPN exhibited the presence of AA. Given that the prevalence of AA is reported to be 1–5% in the general population above the age of 65 years,10,11 JAK2 V617F-positive MPN might be associated with a higher prevalence of AA. In combina- tion with mouse studies, the presence of JAK2 V617F in leukocytes is likely to be a factor of AA development.
The pathogenetic feature of AA is mediated through the degenerative process involving extracellular matrix remodeling in all layers of the arterial wall. Particularly, local activation of inflammatory responses by immune cells plays an important role in the process. Leukocyte accumulation in inflammatory aneurysms provides addi-
tional evidence that inflammation represents variants along a spectrum of aneurysmal disease rather than dis- tinct pathological entities of the inflammatory cells.14 It has been reported that plasma inflammatory cytokine levels in JAK2 V617F-positive MPN are significantly increased in the peripheral blood.42,43 Similarly, we showed that circulating JAK2 V617F leukocytes of MPN patients exhibited significant increases in the expression levels of the cytokines of TGFB3 as well as the chemokines of IL-8. These findings are supported by pre- vious reports that inflammatory macrophages and neu- trophils contributed to AAA development.44,45 It has been reported that macrophage NLRP3 inflammation activa- tion in adventitia promotes inflammatory cytokine pro-
1918
haematologica | 2021; 106(7)