Page 98 - 2021_05-Haematologica-web
P. 98
L. Doll et al.
ABC
DE
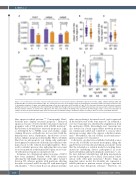
Figure 6. G-csfa induction rescued the reduced neutrophil numbers in the hax1 morphants. (A) Relative expression of hcls1, cebpa, cebpb in wild-type (WT) and morphants (MO) at 2 days post-fertilization (dpf). The b-actin gene was used as an internal control for normalization. N indicates number of biological replicates. (B, C) Quantitative numbers of cebpa- and cebpb-expressing cells in the trunk region of WT and MO at 2 dpf. (D) The top panel illustrates the bi-directional construct (pTGH-g-csfa) used to ectopically induce the zebrafish g-csfa cDNA. Note that green fluorescent protein (GFP) expression was used as a positive control for induction (4 representative embryos are shown in the right panel). The lower panel outlines the timing of the experiment. (E) Fold change of mpo+ cells in the trunk region of embryos at 25 hours post-fertilization (hpf). Each dot represents an individual embryo. N indicates number of embryos. Data are means ± standard deviation.
lular apoptosis-related proteins.15,45 Consequently, Hax1- deficient mice display neuronal apoptosis.15 Increased apoptosis was also observed in the bone marrow myeloid progenitor cells of CN patients with a HAX1 mutation.46 Knockdown of zebrafish hax1 increased apoptosis at 1 dpf, as determined by a TUNEL assay and acridine orange staining. However, cell death was not associated with the hematopoietic tissue. Furthermore, knockdown of hax1 did not enhance apoptosis in neutrophils. Hence, zebrafish Hax1 has an anti-apoptotic role during early embryonic development, but increased cell death is most likely not the main reason for the reduced neutrophil numbers. These results contradict previous data indicating that autosomal recessive mutations in the human HAX1 gene are associat- ed with increased apoptosis in myeloid cells.6
In human, two alternatively spliced isoforms of HAX1 have been identified.4 Patients with HAX1 mutations affecting the full-length transcript or the splice variant I develop CN, whereas patients with mutations affecting the splice isoforms I and II develop CN with neurological abnormalities.5,10 The isoform II uses an alternate in-frame
splice site producing a shortened exon 2, and is expressed in the brain but not in the bone marrow.10 In zebrafish, a sole hax1 transcript was identified by amplification from the embryonic cDNA. Interestingly, hax1 transcript was also detected in a brain region where newborn neurons are continuously added and contribute to sensory infor- mation processing, akin to the superior colliculus in mam- mals.47 Nevertheless, the role of Hax1 in zebrafish neuro- genesis remains to be elucidated.
Collectively, our data have established zebrafish as an in vivo model for HAX1-associated neutropenia, filling the gap between in vitro models and clinical assessment. Given that the zebrafish is a valuable model for studying driver mutations underlying disease pathogenesis in acute myeloid leukemia and myelodysplastic syndromes,48 a sta- ble hax1-deficient zebrafish line will help investigations into the leukemogenic role of Hax1, alone and in combi- nation with other gene mutations.49 Besides being an important cancer model, the zebrafish also represents a reliable platform to perform screens of large compounds and assess their therapeutic relevance.50 Therefore, we
1318
haematologica | 2021; 106(5)