Page 146 - 2021_04-Haematologica-web
P. 146
Y. Shi et al.
A
B
C
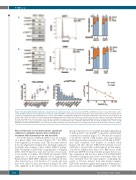
Figure 5. Dasatinib (DAS) inhibits lymphocyte cell-specific kinase (LCK) and leads to cell cycle arrest. (A) SUPT1 and MOLT4 cells were treated with vehicle control or DAS (2 mM) for 24 hours (h) and expression of total LCK, activated p-Y416SRC, total PLCy1 and activated p-Y783PLCγ1 was assessed in western blot analysis (left) or cells were stained with Hoechst and analyzed for cell cycle status (middle and right). (B) Patient-derived xenograft (PDX) LK203 and L963 cells were treated for 24 h with 1 mM of DAS. LCK and PLCγ1 total protein and activating phospho-sites at Y416 and Y783 were assessed in western blot analyses or PDX were stained with Hoechst and analyzed for cell cycle status. (C) The in vitro sensitivity of a panel of T-ALL cell lines to DAS was investigated and IC50 calculated. Phosflow was used to determine the ratio of activated p-Y416SRC/total LCK. This ratio was correlated with in vitro sensitivity to DAS (R2=0.778, P=0.004). Student's t-test: **P<0.01. NB HSB2 was excluded from this analysis, as the extreme sensitivity to DAS was caused by the presence of a unique translocation absent in all other sensitive cell lines.
Phase II-like trial in vivo demonstrates significant reduction in leukemia burden after combination treatment with dexamethasone and dasatinib
To test the efficacy of DEX and DAS in vivo, we conduct- ed a phase II-like trial in mice (Figure 7A).26 Ten PDXs were engrafted in four mice each. The four mice derived from one single patient sample were randomly assigned to treatment arms, namely control vehicle, DEX (1 mg/kg), DAS (35 mg/kg) or DEX+DAS (1 mg/kg DEX + 35 mg/kg DAS). After IF injection, mice tail vein blood was moni- tored weekly for human CD7/CD45 and murine CD45 expression to monitor peripheral blood engraftment. Representative PDX L809 commenced treatment 46 days after injection for a total duration of 3 weeks; the four mice were culled 72 days after injection (Figure 7B). L809 cells engrafted in the spleens of the four mice showed
greatly reduced levels of total LCK and dephosphorylation of LCK (p-Y416SRC and p-Y505LCK) after DAS or DEX+DAS combination treatment (Figure 7C). Western blot analysis of positively selected viable human cells again demon- strated decreased protein expression of LCK and p-Y416SRC after DAS treatment. The number of residual viable human cells after effective DEX+DAS treatment was not sufficient to categorically confirm reduced protein expres- sion (Online Supplementary Figure S7F). One mouse in the DAS arm (LK080) developed uterine prolapse before dos- ing commenced and the mice derived from PDX LK214 succumbed during the first week of treatment. These five were excluded from the final analysis. Combining the results of 35 mice derived from nine patient samples, DEX+DAS treatment significantly impaired leukemia pro- gression more than single drug DEX, DAS or control vehi-
1062
haematologica | 2021; 106(4)