Page 274 - 2021_03-Haematologica-web
P. 274
Case Report
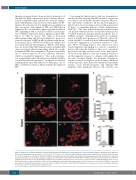
thrombocytopenia derives from an altered interaction of Mk with the ECM components. In fact, mutant Mk pre- sented normal PPF when cultured free from the engage- ment with ECM proteins; however, when plated on fib- rinogen, they showed an abnormally increased adhesion that associated with impaired PPF and SDF1-driven migration. We conclude that a physiologically modulated SRC signalling in Mk is crucial for adhesion and migra- tion on ECM components. In fact, pharmacological inhi- bition of SRC in mouse Mk induced decreased adhesion/spreading and defective migration;7 moreover, reduced activation of SRC in Mk of patients with throm- bocytopenia due to PTPRJ loss-of-function variants also associated with defective migration.13 On the other hand, here we showed that SRC hyperactivation in human Mk causes increased adhesion/spreading, which also results in impaired SDF1-driven migration. The latter is probably due to altered turnover of focal adhesion structures, which is essential for migration.11 Since actin cytoskele- ton reorganization after Mk interaction with the ECM is crucial for proplatelet extension,14 an altered cytoskeletal rearrangement upon Mk adhesion to fibrinogen, due to SRC constitutive activation, likely underlies the impaired PPF.
Concerning the clinical aspects, this case demonstrates that the disorder deriving from SRC mutation can present as isolated, non-syndromic thrombocytopenia. Of note, the only feature common to all the reported patients is platelet anisocytosis and macrocytosis with a proportion of hypo- or agranular platelets (Online Supplementary Table S4).3,4 The other inherited thrombocytopenias that can present with this picture are thrombocytopenia due to GFI1B mutations and gray platelet syndrome due to NBEAL2 variants,15 although in the latter the a-granule defect is usually more pronounced. Therefore, these dis- orders should be considered in the differential diagnosis. A defective aggregation or activation response to colla- gen, albeit of varying degrees, was observed in most reported patients: this finding too could be considered a feature of the disorder. Similar to other cases,3 our patient presented a bleeding tendency more severe than expect- ed based on the platelet count: the a-granule deficiency and the impaired response to collagen could explain the excessive bleeding. Finally, the moderate thrombocy- topenia observed at diagnosis evolved during childhood toward a picture characterized by transfusion-dependent anemia, severe thrombocytopenia, and trilineage BM dysplasia with significant fibrosis. The disorder can
AB
C
D
Figure 2. Analysis of proplatelet formation and spreading in adhesion to fibrinogen of megakaryocytes of the investigated patient. Megakaryocytes (Mk) were incubated on fibrinogen-coated coverslips for 16 hours at 37°C and 5% CO2, fixed and stained with an anti-β1-tubulin antibody (red). Hoechst (blue) was used for counterstaining nuclei. (A-D) Mk of the patient exhibited a markedly increased spreading, often with aberrant morphology, associated with defective extension of typical proplatelets. (A) (iii-vi) Representative examples of patient’s Mk. (i-ii) Proplatelets-forming Mk of healthy controls processed in parallel are shown for comparison. Scale bars: 60 mm. (B) Proplatelet formation was quantified using fluorescence microscopy as the proportion of Mk displaying at least one pro- platelet with respect to the total number of Mk. (C,D) Spreading was measured through image analysis as the average area covered by each Mk (C), and as the percentage of spread Mk with respect to the total number of Mk (D). The data reported with the histograms are expressed as means ± standard deviation. ***P<0.001, and *P<0.05 by two-tailed Student t-test.
920
haematologica | 2021; 106(3)