Page 273 - 2021_03-Haematologica-web
P. 273
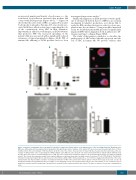
Figure 1. Analysis of maturation and of proplatelet formation in suspension liquid cultures of megakaryocytes of the investigated patient. Megakaryocytes (Mk) were differentiated from peripheral blood progenitors through 14-day culture. Samples of the patient (PT) were processed in parallel with those of three healthy controls (HC). (A-C) Analysis of Mk maturation. (A) At the end of the culture, the proportion of mature Mk was measured by flow cytometry, as the per- centage of CD41-positive cells co-expressing the CD42b antigen.9 (B,C) Mk were also cytospun onto slides and stained with an anti-β1-tubulin antibody (red). Hoechst (blue) was used for counterstaining nuclei (C). Mk were then classified into maturation stages I to IV by morphological analysis according to standard criteria16 (B). Overall, the maturation profile of the patient’s Mk was not different from that of healthy controls. Scale bars: 10 mm. (D-E) Analysis of proplatelet formation in suspension liquid cultures, which measures the intrinsic ability of Mk to form proplatelets i.e., free from the engagement with proteins of the extra- cellular matrix. (D) Representative examples of proplatelet formation of Mk of the patient and controls (phase-contrast microscopy). Scale bars: 10 μm. (E) Proplatelet formation was quantified as the percentage of cells displaying at least one proplatelet with respect to the total number of cells. Overall, the rate of proplatelet formation and the morphology of proplatelets in suspension were similar in patient and controls. The data are expressed as means ± standard devi- ation.
Case Report
an increased number and density of podosomes, i.e., the actin-based focal adhesion structures that mediate Mk contact with ECM proteins (Figure 3A-C).3,10,11 Figure 3A shows that the active form of SRC, recognized by an anti- body specific to phospho-Tyrosine-419, was closely asso- ciated with these adhesion structures, supporting the role of the constitutively active SRC in their formation. Importantly, in adhesion to fibrinogen, an ECM substrate that promotes PPF,8,9 the increased spreading of the patient’s Mk was associated with a significantly reduced extension of typical proplatelets (Figure 2A-B). PPF of mutant Mk adhering to ECM proteins had not been
investigated in previous studies.3,4
Finally, Mk migration on ECM proteins toward a gradi-
ent of stromal cell-derived factor 1 (SDF1) is an essential mechanism for platelet production, as it allows Mk to reach the BM vascular structures in order to release pro- platelets into the circulation.12 Using a modified transwell assay, we found that patient Mk presented a significantly impaired SDF1-driven migration both in adhesion to fib- rinogen and type I collagen (Figure 3D-E).
The study of this patient contributes to elucidate the pathogenesis of SRC-related thrombocytopenia and the role of SRC in human Mk. We provide evidence that
B
D
AC
E
haematologica | 2021; 106(3)
919