Page 120 - 2021_03-Haematologica-web
P. 120
X. Ma et al.
AB
C
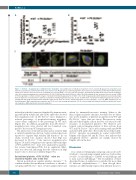
Figure 6. Slc35a1–/– megakaryocytes exhibit defective maturation. (A) Quantification of individual progenitors in bone marrow. Megakaryocyte progenitors were defined as Lin-CD127-Sca1-cKit+CD150+CD41+ bone marrow cells. Megakaryocyte erythroid progenitors were defined as Lin-CD127-Sca1-cKit+CD150+CD41-CD16/32- CD105- bone marrow cells. Data are mean ± standard error of mean (SEM). **P<0.01, n=8 mice/genotype. (B) Polyploidy quantification of bone marrow megakaryo- cytes. Bone marrow megakaryocytes stained with PerCP-Cy5.5-CD41 and Hoechst33342 were subject to ploidy analysis by flow cytometry. Data are means ± SEM. n=8 mice/genotype. (C) Representative images of proplatelet-forming megakaryocytes in CD41+ cells. CD41+ megakaryocytes with cytoplasmic pseudopodia longer than the diameter of cytoplasm were defined as proplatelet-forming megakaryocytes. Bar, 10 mm. Quantification of proplatelet-forming megakaryocytes based on staining with anti-CD41 and anti-β-tubulin antibodies (table below). Data are mean ± standard deviation. n=8 mice/genotype. **P<0.01. MEP, megakaryocyte ery- throid progenitor; MkP, megakaryocyte progenitor; preCFU-E, pre-colony forming unit-erythroid; CFU-E, colony forming unit-erythroid; preGM, pre-granulocytic mono- cytic progenitor; GMP, granulocytic-monocytic progenitor.
induced proplatelet formation identified by immunostain- ing with anti-β-tubulin and anti-CD41 antibodies showed that megakaryocytes in Plt Slc35a1–/– mice displayed a reduced percentage of proplatelet-forming megakary- ocytes when compared to the percentage in WT mice (Figure 6C). In addition, proplatelets from Plt Slc35a1–/– megakaryocytes were shorter and less branched com- pared to those from WT megakaryocytes.
The interaction of thrombopoietin and its receptor Mpl is critical for platelet production. N-glycosylation has been reported to regulate Mpl stability and function.37 In our study, western blot analysis showed that Mpl was decreased in Slc35a1–/– bone marrow megakaryocytes (Figure 7A and B). Megakaryocyte-specific Mpl transcripts of WT and Plt Slc35a1–/– mice were analyzed by quantita- tive reverse transcription PCR, but no significant differ- ence was detected, indicating that the reduction in Mpl may be due to decreased stability (Figure 7C).
Desialylated platelets of Plt Slc35a1–/– mice are cleared by Küpffer cells in the liver
Glycan modifications regulate platelet clearance.7,13 To confirm whether this mechanism contributes to thrombo- cytopenia in Plt Slc35a1–/– mice, we first analyzed liver and
spleen by immunofluorescence staining. Spleen is the major organ for platelet clearance, but there was no differ- ence in the numbers of platelets in spleens from WT and Plt Slc35a1–/– mice (data not shown). Our previous study indicated that Küpffer cells in the liver are critical for the clearance of desialylated platelets.7 We therefore per- formed confocal imaging analysis of liver sections from WT and Plt Slc35a1–/– mice after staining with anti-F4/80 and anti-CD41 antibodies. The results showed that CD41+ Slc35a1–/– platelets are primarily in contact with F4/80+ Küpffer cells (Figure 8A and B). These data indicate that desialylated Slc35a1–/– platelets are mainly cleared by Küpffer cells in the liver.
Discussion
As a common terminal glycosyl group, sialic acid can be attached to non-reducing terminal galactose (Gal) residue via a2,3-, or a2,6-linkage, to GalNAc via a2,6-linkage, and to sialic acid via a2,8-linkage.8,9 The biosynthesis of these diversified forms of sialylation is controlled by more than 20 different sialyltransferases.11,19,38 Even though these enzymes are differentially expressed in different tissues to
766
haematologica | 2021; 106(3)