Page 189 - 2021_02-Haematologica-web
P. 189
ATM-induced mitophagy in MCL
HI
J
K
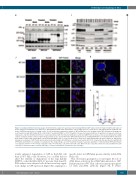
Figure 4. (Continued from the previous page). (H) Immunoblot analysis (30 mg total protein) showing ATM and GFP expression from transient (48 h) and stable (3 weeks) transfection with GFP vector, GFP-LC3 or GFP-Parkin plasmids in WT and Kd HeLa cells. A single blot was cut into pieces and probed with the indicated anti- bodies. GAPDH was probed as a loading control. (I) Cell fractionation immunoblot analysis of WT and Kd HeLa cells showing cellular GFP distribution following tran- sient transfection with GFP-Parkin (48 h). Cells (10x106) treated with DMSO or CCCP (50 mM for 3 h) were fractionated and then 30 mg total or 10 mg each of nuclear, cytoplasmic, and mitochondrial proteins were loaded and probed with the indicated antibodies. Lamin and GAPDH were probed as nuclear and cytoplasmic loading controls. (J) Representative confocal z-plane image analysis (scale: 10 mm) 48 h after GFP-Parkin transfection, showing GFP-Parkin co-localization with the mitochon- drial marker Tom20 in WT and Kd HeLa cells treated with DMSO (Ctrl) or CCCP (50 mM for 3 h). Nuclei were stained with DAPI. (K) Inset of Figure 4J, with arrows showing co-localization of GFP-Tom20 (yellow dots) inside mitochondria after WT HeLa cells had been treated with CCCP. A Laplacian filter was used to identify both GFP-Parkin and Tom20 foci revealing co-localization in the merged image. (L) CCCP treatment resulted in a greater abundance of mitochondrial GFP-Parkin-Tom20 co-localization in WT (****P<0.0001) compared to Kd (*P<0.05) HeLa cells. WT control (68 cells), WT-CCCP (200 cells); Kd control (59 cells) and Kd-CCCP (133 cells) were scanned from three separate passages of cell lines and plotted. (Continued on the next page)
L
icantly enhanced degradation of GFP in Kd-ATM cells than in WT HeLa cells. However, loss of ATM did not affect the stability or degradation of the long half-life (HSP90) or short half-life (MCl-1)35 proteins. Real-time RT- PCR analysis from identical cells did not reveal any signif- icant change in GFP expression (Figure 5D), arguing for a
specific defect in GFP-Parkin protein stability in Kd-ATM HeLa cells.
This observation prompted us to investigate the role of ATM kinase activity in the ATM-Parkin interaction. GFP- Parkin-transfected WT Hela cells were immunoprecipitat- ed with anti-ATM antibody (Figure 5E, F; Online
haematologica | 2021; 106(2)
503