Page 187 - 2021_02-Haematologica-web
P. 187
ATM-induced mitophagy in MCL
AC
B
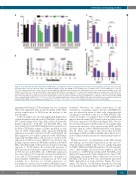
Figure 3. Decreased mitochondrial respiration in shATM mantle cell lymphoma cell lines. (A) High performance liquid chromatography analysis of basal intracellular nucleotide (NTP) levels in untreated mantle cell lymphoma (MCL) control and shATM clones showing mean ± standard error of mean (SEM) (n=3). *P<0.05, **P<0.01: significant differences from respective control shRNA. (B) Graphical representation of the mitochondrial stress test assay in untreated Mino control and shATM clones (8×104 live cells). Treatment with a mitochondrial ATP synthesis uncoupler, FCCP, resulted in the maximal oxygen consumption rate (OCR) by the res- piratory chain. Furthermore, addition of rotenone and antimycin A (complex I and III inhibitors, respectively) prevented the transfer of electrons and thereby dimin- ished OCR, indicating an overall reduction in mitochondria-linked aerobic respiration and ATP production in shATM cells. (C) Relative OCR in untreated MCL shATM clones showing mean ± SEM; (n=3). **P<0.01, ***P<0.001: significant differences from control shRNA. (D) Relative mitochondrial spare respiratory capacity in untreated MCL shATM clones showing mean ± SEM; (n=3). **P<0.01, ***P<0.001, ****P<0.0001: significant differences from control shRNA.
D
unchanged following CCCP treatment, the loss of nuclear ATM and significant gain in mitochondrial ATM likely reflects translocation of ATM from the nucleus to the mitochondria.
Confocal analysis also showed significantly higher mass of mitochondrial nucleoids in Kd-ATM HeLa cells than in WT controls (Figure 4E,F). Since HeLa cells lack detectable Parkin expression,32 both WT and Kd-ATM HeLa cells were transfected with GFP-Parkin plasmid to generate sta- ble HeLa GFP-Parkin isogenic cell lines proficient or defi- cient in ATM. Surprisingly, while transient GFP-Parkin expression was fairly equal in both WT and Kd-ATM cell lines (Figure 4G), we failed to generate stable GFP-Parkin- expressing Kd-ATM HeLa cells. However, neither stable expression of GFP-vector nor GFP-LC3 was affected, sug- gesting a specific defect in GFP-Parkin stability in Kd-ATM HeLa cells (Figure 4H). Cell fractionation studies following exposure to CCCP (Figure 4I) revealed the presence of ATM protein in both nuclear and mitochondrial fractions in WT but not in Kd-ATM cells. GFP-Parkin expression was detected in both cytoplasm and mitochondrial frac- tions, and CCCP treatment enriched the abundance of mitochondrial GFP-Parkin accumulation in WT cells, resulting in a decrease in Tom20 expression via mitophagy. In contrast, mitochondrial GFP-Parkin translo- cation was undetectable in Kd-ATM cells following CCCP
treatment. However, the cellular distribution of the endogenous autophagy adaptor protein, p62/SQSTM1, was not affected in either of these cells, suggesting that autophagy is unrelated to the loss of ATM in HeLa cells. Confocal analysis reconfirmed that CCCP significantly induced mitochondrial GFP-Tom20 double-positive foci in WT cells compared to Kd-ATM cells (Figure 4J-L). We also showed that endogenous Parkin expression is significantly lower in the majority of the early passage MCL shATM cell lines than in control shRNA-transduced cells (Figure 4M, N). These data support the notion that defective mitophagy in MCL and HeLa shATM cells is likely due to lack of Parkin stability and mitochondrial Parkin transloca- tion.
Parkin is known to autoubiquitinate at the UBL domain and undergo proteasomal degradation in the cytosol.33 while endogenous Pink1 is known to be rapidly degraded by UBR1, UBR2 and UBR4 through the “N-end rule path- way”.34 Interestingly, the proteasome inhibitor, MG132 rescued loss of GFP-Parkin stability in Kd-ATM HeLa cells (Figure 5A upper panel; Online Supplementary Figure S5B). Surprisingly, endogenous Pink1 expression was lower in Kd-ATM cells than in WT ones and MG132 prevented Pink1 degradation in both cell lines (Figure 5A lower panel). The kinetics of GFP-Parkin degradation following cycloheximide treatment (Figure 5B, C) suggested a signif-
haematologica | 2021; 106(2)
501