Page 156 - 2021_02-Haematologica-web
P. 156
A. Korporaal et al.
ABCD
EF
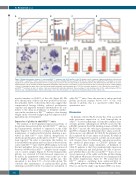
Figure 5. Erythroid progenitor cultures of control and Klf1wt/Nan embryonic day 12.5 fetal liver cells. (A) Growth curves of primary erythroid progenitors cultured from control (Klf1wt/wt) and Klf1wt/Nan E12.5 fetal livers in StemPro medium containing SCF, dex and EPO. (B) Reverse transcriptase quantitative PCR analysis of cell cycle regulators in cultured erythroid progenitors (n=3 for each group). P-values<0.05; error bars indicate standard deviations. (C-D) Differentiation was induced on day 6, transferring the cells to StemPro medium supplemented with EPO and transferrin. Cell number (C) and cell volume (D) was recorded. (E) At day 2 of differentiation cells were centrifuged on glass slides, fixed and stained with dianisidine and histological dyes.32 (F) Flow cytometry analysis of cells cultured from control (Klf1wt/wt) and Klf1wt/Nan fetal livers at day 9 of culture. Cells were stained with antibodies indicated. The percentage of cells double-positive for the erythroid marker CD71 is shown on top, ± standard deviation. (nd): not detectable, due to the virtual absence of expression of myeloid markers on the control cells.
myeloid markers on 20-50% of the cells (Figure 5F). Of note, the majority of these cells were positive for the ery- throid marker CD71. Collectively, these data suggest that compromised lineage fidelity, reduced proliferative capacity and impaired terminal differentiation all con- tribute to the delay in abundance of definitive erythro- cytes in the circulation of Klf1wt/Nan embryos, thus bearing weight on the observed changes in globin expression dur- ing embryonic development.
Expression of globins in adult Klf1wt/Nan mice
The analysis of developmental expression patterns of the globins demonstrated that by E16.5 Klf1wt/Nan embryos had quantitatively switched to expression of the adult genes (Figures 1-3). However, it remains possible that the maintenance of embryonic/fetal globin silencing is per- turbed in adult Klf1wt/Nan mice. Indeed, derepression of embryonic globin genes in the spleen of Klf1wt/Nan mice has been reported.24 In order to investigate this further, we isolated RNA from spleen and bone marrow derived from control and Klf1wt/Nan mice. By RT-qPCR analysis we found that mz and mβh1, but not me, expression was increased between 35-800-fold in Klf1wt/Nan samples check compari- son to control samples (Figure 6). For the human β-like globins, we observed 4-9-fold increased expression of he and hγ. In quantitative terms, even in the case of the most highly expressed embryonic globin mz, this amounted to less than 0.3% of total α-like globin. We conclude that maintenance of embryonic/fetal globin silencing is perturbed in the bone marrow and spleen of
adult Klf1wt/Nan mice. Since the amount of embryonic/fetal globins produced remains below 0.3% of the total amount of globins, this is a qua-litative rather than a quantitative trait.
Discussion
In humans, reduced KLF1 activity has been associated with persistent expression of fetal hemoglobin in adults.1,14 A severe phenotype of hemolytic anemia char- acterizes patients suffering from CDA-IV, caused by the p.E325K variant in the DNA binding domain of KLF1. In the Klf1wt/Nan mouse, the orthologous glutamic acid residue (p.E339) is changed. Biochemically, these amino acid sub- stitutions are very different. In CDA-IV, the glutamic acid (E) is replaced by a basic amino acid (lysine, K) while in KLF1Nan it is replaced by aspartic acid (D), an acidic amino acid. Despite these biochemically opposing properties, the erythroid phenotypes of CDA-IV patients and Klf1wt/Nan mice share many similarities. A hallmark of CDA-IV patients is that they maintain high expression levels of embryonic and fetal globins. In order to investi- gate whether this is also the case in Klf1wt/Nan mice, we sur- veyed expression of the α-like and β-like globins during development. Our main observations are summarized in the Online Supplementary Figure S1. We found that in Klf1wt/Nan embryos, switching from embryonic/fetal to adult globin genes is delayed in the endogenous Hbb and Hba loci, and in the single-copy human HBB locus trans-
470
haematologica | 2021; 106(2)