Page 69 - 2020_09-Haematologica-web
P. 69
XPO1 is a target to treat β-thalassemia
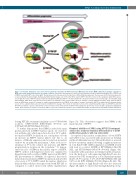
Figure 5. Schematic illustration of the molecular mechanisms modulated by KPT treatment in β-thalassemia major (β-TM) erythroid progenitors compared to β-TM and normal erythroid progenitors in normal conditions. Schematic representation of molecular mechanisms in normal erythroid progenitor (EP), β-TM EP, and β-TM EP treated with KPT (cells on the right). The big arrow on the top represents the direction of differentiation progression. The decrease in XPO1 protein expression is represented by the pink triangle. During early differentiation stages (cell on the left), XPO1 exports HSP70 from the nucleus to the cytoplasm, while XPO1 expres- sion is high. The entry of HSP70 being constant and mediated by Hikeshi, HSP70 is localized both in the cytoplasm and the nucleus at this stage. In normal EP, along differentiation, while XPO1 expression decreases, HSP70 accumulates in the nucleus until caspase-3 activates, corresponding to basophilic stage. Nuclear HSP70 protects GATA1 from caspase-3 cleavage to enable terminal maturation. In β-TM EP, at the stage of caspase-3 activation, HSP70 is trapped in the cytoplasm by the excess of free a-globin chains and can not protect GATA1 from cleavage. This results in maturation arrest at the polychromatophilic stage. In β-TM EP treated with KPT, XPO1 activity is repressed. This allows nuclear retention of the small amount of HSP70 that managed to get into the nucleus despite cytoplasm trapping by α chains. At the moment of caspase-3 activation, HSP70 is present in sufficient amount to protect GATA1 and enable an improvement in β-TM EP terminal maturation.
lowing KPT-251 treatment [similarity score 0.7483±0.006 (control), 0.8587±0.005 (KPT100nM) (P<0.01) and 0.9872±0.005 (KPT1000nM) (P<0.01)].
To further demonstrate that XPO1 is indeed the main protein involved in HSP70 nuclear export, we tested its role in HeLa cells, which upon heat shock at 43°C exhib- ited nuclear HSP70 localization as a consequence of both an increase in HSP70 nuclear inflow rate due to an increase of Hikeshi expression and a reduction in nuclear outflow by an unknown mechanism.24 After a 6-h recov- ery phase at 37°C, the outflow rate increases and HSP70 progressively re-localizes in the cytoplasm.24 To demon- strate the role of XPO1 in the nuclear export of HSP70, HSP70 outflow following heat shock was analyzed by ImageStream with or without XPO1 repression (24 h KPT- 251 pre-treatment at 1000nM). As expected, heat shock induced increased nuclear HSP70 localization and after 6 h of recovery at 37°C, HSP70 exited the nucleus, which was delayed when cells have been pre-treated with KPT-251 as compared to control treated cells (Online Supplementary
Figure S3). This observation suggests that XPO1 is the main exportin of HSP70.
Chemical inhibition of XPO1 using KPT-251 treatment ameliorates erythroid terminal differentiation of β-TM erythroid progenitors with low cytotoxicity
As expected, this nuclear accumulation of both HSP70 and GATA-1 was associated with an increase in terminal erythroid differentiation of β-TM erythroblasts as assessed by flow cytometry analysis showing a significant increase in total Band3 MFI9 [MFI normalized on DMSO treated cells used as a control: DMSO=1, KPT100nM=1.06±0.04 (NS), and KPT1000nM=1.48±0.09 (P<0.01)] (Figure 4A). Consistent with an increase in terminal erythroid matura- tion, the fraction of cells expressing high Band3 was also increased by KPT treatment in a dose-dependent manner [2.62%±0.39 (control), 3.13%±0.53 (KPT100nM) (NS) and 9.27%±1.44 (KPT 1000nM) (P<0.01)] (Figure 4B, left). The absolute number of high Band3 cells was also increased (Figure 4B, right). To ensure that this high Band3 pool rep-
haematologica | 2020; 105(9)
2247