Page 159 - 2020_08-Haematologica-web
P. 159
Attenuating leukemia chemoresistance in the CNS
cytarabine in reducing the number of viable leukemia cells in the meninges (Figure 6D-G). Moreover, Me6TREN sig- nificantly extended the survival of mice treated with cytarabine in the patient-derived xenotransplantation model (Online Supplementary Figure S8). Despite Me6TREN disrupting the bone marrow hematopoietic niche,38 mice receiving cytarabine/Me6TREN or cytara- bine alone exhibited comparable hematologic toxicities (Online Supplementary Figure S9). Finally, disruption of the CNS leukemia niche with Me6TREN did not result in an increased leukemia burden in other organs or tissues (Online Supplementary Figure S10).
We then performed gene expression profiling on pri- mary, human meningeal cells treated with Me6TREN in order to take an unbiased approach toward identifying the mechanisms by which Me6TREN disrupts leukemia- meningeal adhesion. As predicted, pathway analyses identified cell adhesion and migration as being among the most differentially regulated in meningeal cells treated with Me6TREN (Online Supplementary Figure S11A and Online Supplementary Table S2). The gene expression data also showed that Me6TREN significantly downregulated the cell-surface/adhesion proteins VCAM-1 and CD99 (Online Supplementary Figure S11B). Supporting a function- al role for CD99 and VCAM-1 in leukemia-meningeal
adhesion we found that antibodies targeting either of these proteins attenuated the adhesion of leukemia and meningeal cells (Online Supplementary Figure S11C, D). We also examined matrix metalloproteases (MMP) because Me6TREN has been shown to upregulate MMP-9 in the context of hematopoietic stem cells and the bone marrow niche.38 Supporting a similar role in the CNS niche, two different MMP inhibitors diminished the ability of Me6TREN to disrupt leukemia-meningeal adhesion (Online Supplementary Figure S11C, D). In contrast, Me6TREN did not significantly perturb the CXCR4 and CCR7 signaling pathways, which have been previously implicated in leukemia infiltration into the CNS (Online Supplementary Figure S12 and Online Supplementary Table S3).39,40
Discussion
Protection from leukemia relapse in the CNS is crucial to long-term survival and quality of life of patients with leukemia.1-3 One strategy for developing novel CNS- directed therapies has focused on identifying, and poten- tially targeting, the factors that facilitate leukemia cell traf- ficking to the CNS from the bone marrow.39,41-49 At the
A
BC
DEF
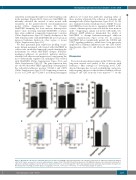
Figure 3. Meningeal cells increase leukemia quiescence in vitro. (A, B) NALM-6 and Jurkat leukemia cells cultured in suspension or adherent to primary meningeal cells for 48 h were assessed for cell cycle and proliferation using a Click-iT Plus EdU kit and flow cytometry. (C-F) NALM-6 leukemia cells cultured in suspension or adherent to primary meningeal cells for 48 h were stained for Ki-67 (C) or Hoechst-pyronin Y (D-F) and analyzed by flow cytometry. Representative flow plots for Hoechst-pyronin Y staining are shown (D-E). For all graphs, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 by analysis of variance.
haematologica | 2020; 105(8)
2135