Page 223 - Haematologica - Vol. 105 n. 6 - June 2020
P. 223
FHL2 modulates thrombosis formation
AC
BD
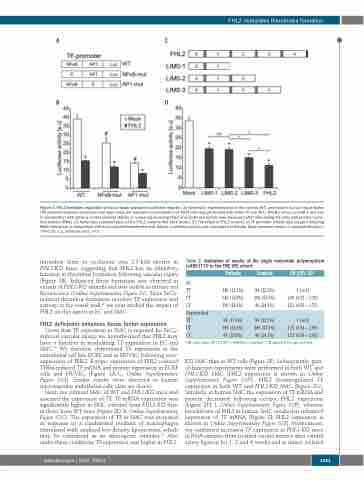
Figure 3. FHL2-mediated regulation of tissue factor promoter luciferase reporter. (A) Schematic representation of the normal (WT) and mutant human tissue factor (TF) promoter-reporter constructs that were used. (B) Transient co-transfection of 293T cells was performed with either TF-Luc (WT), NFκB mut-Luc and AP-1 mut-Luc in combination with either a control plasmid (Mock) or a plasmid encoding FHL2 and luciferase activity was measured after stimulating the cells with phorbol myris- tate acetate (PMA). (C) Schematic representation of the FHL2 variants that were tested. (D) The effect of FHL2 variants on TF promoter activity was assayed following PMA stimulation in comparison with mock-plasmid transfected cells (Mock). Luciferase activity was normalized to Renilla. Data represent means ± standard deviation. *P<0.05. a.u, arbitrary units. n=3
thrombus (time to occlusion) was 2.3-fold shorter in FHL2-KO mice, suggesting that FHL2 has an inhibitory function in thrombus formation following vascular injury (Figure 1B). Enhanced fibrin formation was observed in vessels of FHL2-KO animals and was visible as intense red fluorescence (Online Supplementary Figure S1). Since FeCl3- induced thrombus formation involves TF expression and activity in the vessel wall,39 we next studied the impact of FHL2 on this aspect in EC and SMC.
FHL2 deficiency enhances tissue factor expression
Given that TF expression in SMC is required for FeCl3- induced vascular injury, we hypothesized that FHL2 may have a function in modulating TF expression in EC and SMC.40 We therefore determined TF expression in the endothelial cell line ECRF and in HUVEC following over- expression of FHL2. Ectopic expression of FHL2 reduced TNFα-induced TF mRNA and protein expression in ECRF cells and HUVEC (Figure 2A-C; Online Supplementary Figure S2A). Similar results were observed in human microvascular endothelial cells (data not shown).
Next, we cultured SMC of WT and FHL2-KO mice and assessed the expression of TF. TF mRNA expression was significantly higher in SMC cultured from FHL2-KO than in those from WT mice (Figure 2D-E; Online Supplementary Figure S2C). The expression of TF in SMC was increased in response to a conditioned medium of macrophages stimulated with oxidized low density lipoproteins, which may be considered as an atherogenic stimulus.33 Also under these conditions TF expression was higher in FHL2-
Table 2. Validation of results of the single nucleotide polymorphism rs4851770 in the THE VTE cohort.
All
TT
CT
CC Unprovoked TT
CT
CC
Patients
145 (21.5%) 340 (50.4%) 190 (28.1%)
58 (17.3%)
180 (53.6%)
98 (29.2%)
Controls
84 (22.5%) 200 (53.5%) 90 (24.1%)
84 (22.5%)
200 (53.5%)
90 (24.1%)
OR (95% CI)*
1 [ref]
1.00 (0.72 – 1.38) 1.21 (0.83 – 1.75)
1 [ref]
1.25 (0.82 – 1.89)
1.53 (0.96 – 2.45)
OR: odds ratio; 95% CI: 95% confidence interval. *OR adjusted for age and sex.
KO SMC than in WT cells (Figure 2F). Subsequently, gain- of-function experiments were performed in both WT and FHL2-KO SMC (FHL2 expression is shown in Online Supplementary Figure S2D). FHL2 downregulated TF expression in both WT and FHL2-KO SMC (Figure 2G). Similarly, in human SMC the expression of TF mRNA and protein decreased following ectopic FHL2 expression (Figure 2H, J; Online Supplementary Figure S2B), whereas knockdown of FHL2 in human SMC resulted in enhanced expression of TF mRNA (Figure 2I; FHL2 expression is shown in Online Supplementary Figure S2E). Furthermore, we confirmed increased TF expression in FHL2-KO mice in RNA samples from isolated carotid arteries after carotid artery ligation for 1, 2 and 4 weeks and in intact, isolated
haematologica | 2020; 105(6)
1681