Page 224 - Haematologica - Vol. 105 n. 6 - June 2020
P. 224
C. Kroone et al.
mouse aorta to rule out confounding tissue culture-related effects (Online Supplementary Figure S3A, B). We also demonstrated that bone-marrow derived macrophages from FHL2-KO mice showed increased mRNA levels of TF under basal, lipopolysaccharide-, and interleukin-4- stimulated conditions compared to macrophages derived from WT mice (Online Supplementary Figure S3C). Together, these data indicate that FHL2 inhibits TF expres- sion in EC, SMC and macrophages.
FHL2 regulates tissue factor expression through inhibition of nuclear factor κB and activating protein-1 FHL2 does not bind DNA directly but is known to affect the activity of genuine transcription factors such as NFκB and AP-1. The TF promoter contains functional binding motifs for NFκB and AP-1.25,26 To elucidate the mechanism by which FHL2 suppresses transcription of the TF gene, we performed transient transfections with WT and mutant TF promoter-reporter constructs in HEK293T cells
AB
CD
EFGH
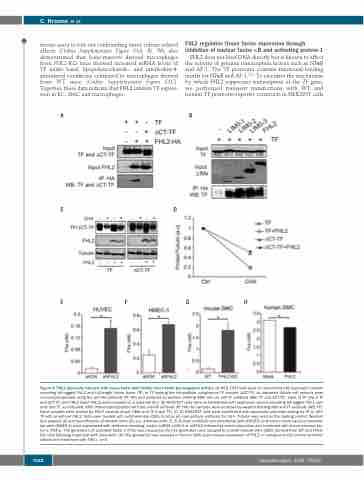
Figure 4. FHL2 physically interacts with tissue factor and inhibits tissue factor pro-coagulant activity. (A) HEK 293T cells were co-transfected with expression vectors encoding HA-tagged FHL2 and full-length tissue factor (TF) or TF lacking the intracellular cytoplasmic TF domain (∆CT-TF), as indicated. Whole cell extracts were immunoprecipitated using the anti-HA antibody (IP: HA) and analyzed by western blotting (WB) with an anti-TF antibody (WB: TF and ∆CT-TF). Input of TF (Input TF and ∆CT-TF) and FHL2 (Input FHL2) were revealed on a separate blot. (B) HEK293T cells were co-transfected with expression vectors encoding HA-tagged FHL2 vari- ants and TF, as indicated. After immunoprecipitation with the anti-HA antibody (IP: HA) the samples were analyzed by western blotting with anti-TF antibody (WB: TF). Input samples were probed for FHL2 variants (Input LIMs) and TF (Input TF). (C, D) HEK293T cells were transfected with expression plasmids coding for TF or ∆CT- TF with or without FHL2. Cells were treated with cycloheximide (CHX) to block de novo protein synthesis for 16 h. Tubulin was used as the loading control. Western blot analysis (C) and quantification of western blots (D); a.u, arbitrary units. (E, F) Human umbilical vein endothelial cells (HUVEC) and human micro-vascular endothe- lial cells (HMEC-1) were transduced with lentivirus encoding control shRNA (shCtrl) or shFHL2 followed by serum-starvation and treatment with tumor necrosis fac- tor-α (TNFα). The generation of activated factor X (FXa) was measured. (G) FXa generation was assayed in smooth muscle cells (SMC) derived from WT and FHL2- KO mice following treatment with ionomycin. (H) FXa generation was assayed in human SMC upon ectopic expression of FHL2 or transduced with control lentivirus (Mock) and treatment with TNFα. n=3.
1682
haematologica | 2020; 105(6)