Page 207 - Haematologica - Vol. 105 n. 6 - June 2020
P. 207
GpIb clustering in shear-activated platelets
functional side, this provides an explanation as to why adhesion is significantly inhibited by ALA pre-treatment while platelet rolling speed is increased (Figure 1A, C). Previous work has shown that ω-3 FA (of marine origin) can inhibit protein palmitoylation and, therefore, localiza- tion to lipid rafts.35 Although in our experiments the pre- incubation time was too short to achieve an analog effect, long-term, nutritional supplementation with ALA may also inhibit GpIb localization to lipid rafts via reduced
palmitoylation and, consequently, reduce platelet adhe- sion to vWF even more through this additional mecha- nism.
Taken together, these data provide insight into the pos- sible mechanism of the anti-thrombotic properties of n-3 FA in the early phase of thrombosis at sites of arterial stenosis or plaque. It may therefore represent the basis for a therapeutic approach that interferes with this process.
In conclusion, our structural data from intact platelets,
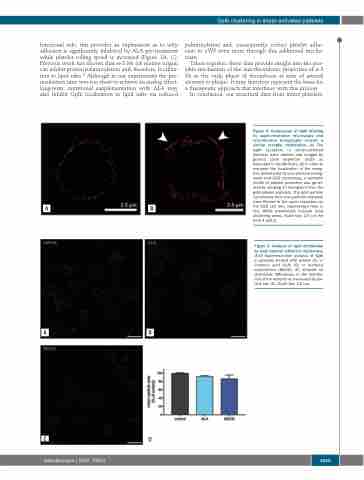
Figure 4. Comparison of GpIb labeling by super-resolution microscopy and cryo-electron tomography reveals a similar receptor distribution. (A) The GpIb receptors in shear-activated platelets were labeled and imaged by ground state depletion (GSD) as described in the Methods. (B) In order to compare the localization of the recep- tors determined by cryo-electron tomog- raphy and GSD microscopy, a synthetic model of platelet perimeter was gener- ated by merging 17 tomograms from the gold-labeled platelets. The gold particle coordinates from the synthetic platelets were filtered to the same resolution as the GSD (20 nm), represented here in red. White arrowheads indicate local clustering areas. Scale bar: 2.5 μm for both A and B.
Figure 5. Analysis of GpIb distribution by total internal reflection microscopy. (A-D) Superresolution analysis of GpIb in platelets treated with vehicle (A), α- linolenic acid (ALA) (B) or methyl-β cyclodextrin (MbCD) (C) showed no detectable differences in the distribu- tion of the receptor as measured by par- ticle size (D). Scale bar: 2.5 μm.
AB
AB
CD
haematologica | 2020; 105(6)
1665