Page 205 - Haematologica - Vol. 105 n. 6 - June 2020
P. 205
GpIb clustering in shear-activated platelets
AB
C
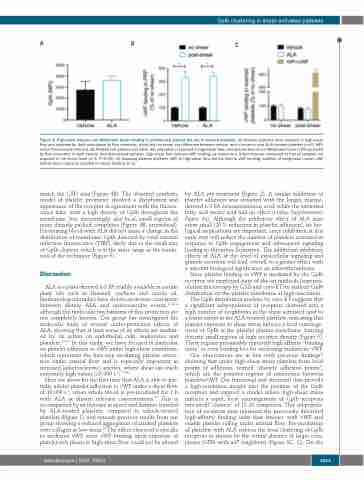
Figure 2. High-shear induces von Willebrand factor binding in platelet-rich plasma but not in washed platelets. (A) Washed platelets were exposed to high-shear flow and analyzed for GpIb abundance by flow cytometry, which did not reveal any difference between vehicle- and α-linolenic acid (ALA)-treated platelets (n=3). MFI: mean fluorescence intensity. (B) Platelet-rich plasma was either left untreated or exposed to high-shear flow, and platelet-bound von Willebrand factor (vWF) analyzed by flow cytometry. In both vehicle- and ALA-treated samples, high-shear flow induced vWF binding, as shown by a 3-fold increase compared to that of samples not exposed to the shear force (n=3, P<0.05). (C) Exposing washed platelets (WP) to high-shear flow did not lead to vWF binding; addition of exogenous human vWF before shear exposure resulted in robust binding (n=3).
match the GSD data (Figure 4B). The obtained synthetic model of platelet perimeter showed a distribution and appearance of the receptor in agreement with the fluores- cence data, with a high density of GpIb throughout the membrane, but, interestingly, also local, small regions of more densely packed complexes (Figure 4B, arrowhead). Pre-treating blood with ALA did not cause a change in the distribution of membrane GpIb detected by total internal reflection fluorescence (TIRF), likely due to the small size of GpIb clusters, which is in the same range as the resolu- tion of the technique (Figure 5).
Discussion
ALA is a plant-derived n-3 FA readily available in certain plant oils such as flaxseed, soybean and canola oil. Epidemiological studies have shown an inverse correlation between dietary ALA and cardiovascular events,11,30,31 although the molecular mechanisms of this protection are not completely known. Our group has investigated the molecular basis of several cardio-protective effects of ALA, showing that at least some of its effects are mediat- ed by its action on endothelial cells, leukocytes and platelets.9,10,19 In this study, we have focused in particular on platelet adhesion to vWF under high-shear conditions, which represents the first step mediating platelet activa- tion under arterial flow and is especially important in stenosed (atherosclerotic) arteries, where shear can reach extremely high values (>5,000 s-1).7,32,33
Here we show for the first time that ALA is able to par- tially inhibit platelet adhesion to vWF under a shear flow of 10,000 s-1, when whole blood is pre-incubated for 1 h with ALA at dietary relevant concentrations.23 This is accompanied by an increase in speed and distance traveled by ALA-treated platelets, compared to vehicle-treated platelets (Figure 1), and extends previous results from our group showing a reduced aggregation of citrated platelets over collagen at low shear.19 The effect observed is specific to anchored vWF, since vWF binding upon exposure of platelet-rich plasm to high-shear flow could not be altered
by ALA pre-treatment (Figure 2). A similar inhibition of platelet adhesion was obtained with the longer, marine- derived n-3 FA eicosapentaenoic acid, while the saturated fatty acid stearic acid had no effect (Online Supplementary Figure S4). Although the inhibitory effect of ALA may seem small (25% reduction in platelet adhesion), its bio- logical implications are important, since inhibition at this early step will reduce the number of platelets activated in response to GpIb engagement and subsequent signaling leading to thrombus formation. The additional inhibitory effects of ALA at the level of intracellular signaling and granule secretion will lead, overall, to a greater effect with a relevant biological significance on atherothrombosis.
Since platelet binding to vWF is mediated by the GpIb receptor, we employed state-of-the-art methods (superres- olution microscopy by GSD and cryo-ET) to analyze GpIb distribution on the platelet membrane at high-resolution.
The GpIb distribution analysis by cryo-ET suggests that a significant subpopulation of receptors clustered into a high number of neighbours in the shear activated (and to a lesser extent in the ALA-treated) platelets, indicating that platelet exposure to shear stress induces a local rearrange- ment of GpIb at the platelet plasma membrane, forming discrete small regions of high receptor density (Figure 3). These regions presumably represent high affinity “binding units” or even binding loci for anchoring multimeric vWF.
Our observations are in line with previous findings34 showing that under high-shear stress platelets form local points of adhesion, termed “discrete adhesion points”, which are the putative regions of interaction between platelets/vWF. Our functional and structural data provide a high-resolution insight into the position of the GpIb receptors and support a model where high-shear stress induces a rapid, local rearrangement of GpIb receptors into small “clusters” of 15-20 complexes. This subpopula- tion of receptors may represent the previously described high-affinity binding units that interact with vWF and enable platelet rolling under arterial flow. Pre-incubation of platelets with ALA reduces the local clustering of GpIb receptors as shown by the virtual absence of larger com- plexes (GPIb with ≥17 neighbors) (Figure 3C, G). On the
haematologica | 2020; 105(6)
1663