Page 50 - Haematologica May 2020
P. 50
H. Li et al.
stantially increased accordingly (Figure 5B and C). Proteomic analysis was performed to investigate the mechanisms behind the EGR1 knockdown-induced increased proliferation. Of a total of 4,520 identified pro- teins, 190 were differentially expressed between EGR1 knockdown stromal cells and scramble and non-transfect- ed controls (Online Supplementary Tables S4 and S5 and Online Supplementary Figure S4E-G). A group of ten pro- teins (HSD17B11, UQCRC1, CYP1B1, NDUFA8, TXNDC17, CYCS, FADS3, PDIA5, PCYOX1, QSOX1) related to oxidative-reduction processes was expressed lower in EGR1 knockdown cells (Figure 6A and B) as were GLS1, GPX1 and GSTP1, which are also associated with intracellular reactive oxygen species (ROS) accumula- tion.15-17 Accordingly, ROS levels in EGR1 knockdown cells
were increased compared to controls (Figure 6C and D). Inhibiting ROS using an antioxidant cocktail (L-ascorbic acid and citric acid), N-acetylcysteine (NAC), or apocynin considerably reduced ROS production in EGR1 knock-
down cells (Figure 6E). ROS reduction caused a decrease in the fraction of dividing EGR1 knockdown cells (Figure 6F) and in the numbers of CFU-F with complete abroga- tion of colony formation when using the antioxidant cock- tail (Figure 6G). In contrast, neither myeloperoxidase (MPO) inhibitor 4-ABAH nor the small molecule inhibitor LY2228820 (Figure 6E-G) affected ROS levels, percentages of proliferating cells or CFU-F numbers.
Consistent with the increased proliferation in EGR1- knockdown BMSC, gene expression profiling identified a group of down-regulated genes involved in cell death and apoptosis (BFAR, EIF4G2, TSPO, RABEP1, MCL1, TNFRSF12A, TBRG4, MYC, DDIT4) in EGR1 knockdown cells (Online Supplementary Table S2 and Online Supplementary Figure S5B and C). Furthermore, positive regulators of cell proliferation and negative regulators of apoptosis were down-regulated in EGR1 over-expressing cells (Online Supplementary Table S3 and Online Supplementary Figure S5D), which is consistent with the
A
B
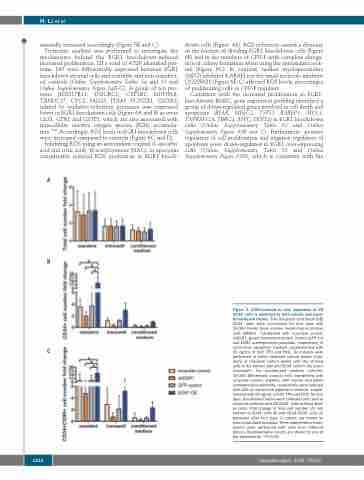
Figure 3. EGR1-induced ex vivo expansion of CB CD34+ cells is mediated by both soluble and mem- brane-bound factors. Five thousand cord blood (CB) CD34+ cells were co-cultured for four days with 10,000 feeder bone marrow mesenchymal stromal cells (BMSC) transfected with scramble control, shEGR1, green fluorescent protein control (GFP ctr) and EGR1 overexpression plasmids, respectively, in serum-free expansion medium supplemented with
C 25 ng/mL of SCF, TPO and Flt3L. Co-cultures were performed in either standard culture plates (stan- dard) or transwell culture plates with the stromal cells in the bottom well and CD34+ cells in the insert (transwell). For conditioned medium cultures, 10,000 BM-derived stromal cells transfected with scramble control, shEGR1, GFP control and EGR1 overexpression plasmids, respectively, were cultured with 200 mL serum-free expansion medium supple- mented with 25 ng/mL of SCF, TPO and Flt3L for four days. Conditioned media were collected and used to stimulate cultures with CB CD34+ cells (without feed- er cells). Fold change of total cell number (A), cell number of CD34+ cells (B) and CD34+CD90+ cells (C) produced after four days in culture are shown as mean±standard deviation. Three independent exper- iments were performed with cells from different donors. Representative results are shown for one of
the experiments. *P<0.05.
1210
haematologica | 2020; 105(5)