Page 52 - Haematologica May 2020
P. 52
H. Li et al.
functions are not well known. Murine studies using inducible gene deletion strategies in BMSC indicated a tight control of key BMSC functions to maintain home- ostasis of the hematopoietic and the skeletal system.18, 19 Here we report for the first time that EGR1 is a key regu- lator in human BMSC.
EGR1 is a member of the immediate early response transcription factor family with a role in regulating devel- opment, growth control and stress response in many tis- sues. Based on our EGR1 expression data in primary BMSC,1 and information on the role of EGR1 in stromal cell growth factor production regulation and hematopoiet- ic stem cell regulation,6,20 we hypothesized that EGR1 could also be an important regulator of BM stromal stem cells.
In accordance with our previous data1 we found that EGR1 expression was substantially higher in highly CFU- F-enriched primary lin–CD45–CD271+CD140a– BMSC (Figure 1A). In addition, EGR1 was significantly higher expressed in steady-state adult BMSC in comparison to fetal BMSC and BMSC in regenerating marrow (Figure 1B), which have higher proliferation rates as indicated by high cell cycle activator expression levels in fetal BMSC (data not shown) and previously reported proliferation data.21,22 Moreover, EGR1 was the only member of the EGR family (EGR1-4) that was highly expressed in BMSC (Online Supplementary Figure S1A and B), in contrast to other cell types that also co-express other EGR family members.23,24
These data implied that EGR1 expression levels might be connected to the regulation of key BMSC functions, i.e. hematopoietic support in steady-state and stroma prolifer- ation in situations with regenerative demands. In fact, EGR1 overexpression increased BMSC hematopoietic stroma-supporting function and facilitated the effective generation of transplantable hematopoietic stem cells, while EGR1 knockdown abrogated the stroma contribu- tion in HSC expansion co-cultures (Figure 2). Furthermore, a clear reduction in ex vivo expanded HSC was recorded in transwell cultures and when using condi- tioned medium (Figure 3). Our data thus indicated that EGR1 regulated BMSC stroma support functions mediat- ed by both cell-cell contact and soluble factors, both of which are required to realize effective ex vivo HSC expan-
sion.25-28 Accordingly, gene array expression profiling iden- tified hematopoiesis-supporting genes as EGR1 down- stream targets, which corresponded to both secreted and surface-expressed molecules (Figure 4A). Of these, we investigated the functional role of CCL28 and VCAM1 as examples for a potent niche-secreted soluble growth fac- tor11 and an adhesion molecule, respectively. Here, CCL28 supplementation and blocking experiments (Figure 4D-I) clearly indicated a contributing role of CCL28 in EGR1- mediated ex vivo expansion of CB CD34+ cells on EGR1 over-expressing stromal cells. Similarly, the role of VCAM1 was demonstrated by blocking VCAM1 with neutralizing antibodies (Figure 4G-I).
Successful ex vivo expansion of HSC represents a prom- ising approach to provide sufficient numbers of trans- plantable stem cells and to facilitate the development of cell and gene therapies. A number of approaches to expand HSC have been pursued, including enhancing pos- itive signals and inhibiting negative signals for HSC self- renewal.29,30 Our results indicate that modification of feed- er cell regulation by EGR overexpression represents a novel approach to generate an optimized microenviron- ment that supports HSC self-renewal and growth with the potential to improve current HSC expansion culture con- ditions. Furthermore, one can envision strategies to increase EGR1 expression in vivo to improve hematopoiet- ic stromal support function, for example, in transplanta- tion patients with poor graft function, and possibly even in patients with insufficient hematopoiesis, such as low- risk myelodysplastic syndrome.
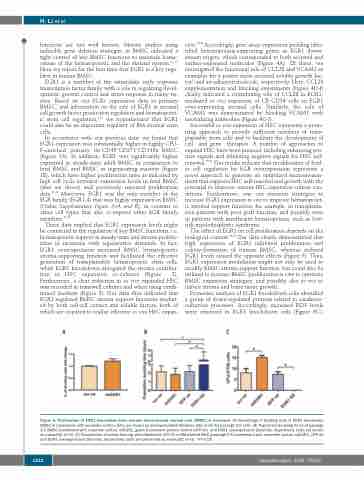
The effect of EGR1 on cell proliferation depends on the biological context.31,32 Our data clearly demonstrated that high expression of EGR1 inhibited proliferation and colony-formation of human BMSC, whereas reduced EGR1 levels caused the opposite effects (Figure 5). Thus, EGR1 expression modulation might not only be used to modify BMSC stroma support function, but could also be utilized to increase BMSC proliferation in vitro to optimize BMSC expansion strategies, and possibly also in vivo to induce stroma and bone tissue growth.
Proteomic analysis of EGR1 knockdown cells identified a group of down-regulated proteins related to oxidative- reduction processes. Accordingly, increased ROS levels were observed in EGR1 knockdown cells (Figure 6C),
ABC
Figure 5. Proliferation of EGR1 knockdown bone marrow mesenchymal stromal cells (BMSC) is increased. (A) Percentage of dividing cells in EGR1 knockdown BMSC in comparison with scramble control. Data are shown as mean±standard deviation (SD) (n=5) for passage 2-5 cells. (B) Population doubling times of passage 2-5 BMSC transfected with scramble control, shEGR1, green fluorescent protein control (GFP ctr) and EGR1 overexpression plasmids, respectively. Data are shown as mean±SD (n=3). (C) Frequencies of colony-forming units-fibroblasts (CFU-F) in BM-derived MSC (passage 2-5) transfected with scramble control, shEGR1, GFP ctr and EGR1 overexpression plasmids, respectively. Data are presented as mean±SD (n=3). *P<0.05.
1212
haematologica | 2020; 105(5)