Page 242 - Haematologica May 2020
P. 242
P.J. Teoh et al.
A
B
To date, 1q21(amp) has remained a high-risk prognostic factor in MM. This poses a serious hurdle in myeloma management since a high percentage of newly diagnosed patients present with this abnormality and they are reported to be resistant to standard and novel thera- pies.16,17,39,40 Although the clinical significance of CKS1B, the most commonly reported candidate driver gene,18,39 has already been established, we believe that other genes within the amplified region may also be of pathogenic importance. Here, we impart novel information about the pathogenesis of 1q21(amp) and provide compelling evi- dence that IL6R and ADAR1 on chromosome 1q21 are crit- ical genes for myeloma pathogenesis. Concomitant gain of their genomic loci conferred growth and proliferation privilege to MM cells, working through hyperactivation of the STAT3 pathway. With regards to ADAR1, we saw an isoform-predominant phenomenon in which the P150 iso- form was found to be intertwined in the STAT3 pathway
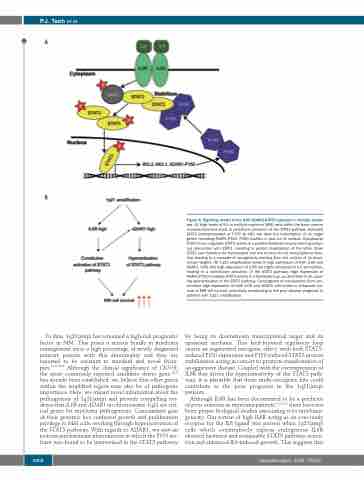
Figure 8. Signaling model of the IL6R-ADAR1-STAT3 interplay in multiple myelo- ma. (A) High levels of IL6 in multiple myeloma (MM) cells within the bone marrow microenvironment leads to persistent activation of the STAT3 pathway. Activated STAT3 (phosphorylated at Y705 by JAK) will drive the transcription of its target genes, including ADAR1-P150. P150 shuttles in and out of nucleus. Cytoplasmic P150 in turn regulates STAT3 activity in a positive feedback loop by forming a phys- ical interaction with STAT3, resulting in protein stabilization of the latter. More STAT3 can therefore be translocated into the nucleus for its transcriptional func- tion, leading to a cascade of oncogenicity deriving from the actions of its down- stream targets. (B) 1q21 amplification leads to high expression of both IL6R and ADAR1. Cells with high expression of IL6R are highly sensitized to IL6 stimulation, leading to a constitutive activation of the STAT3 pathway. High expression of ADAR1-P150 mediates STAT3 activity in a feedback loop, as described in (A), caus- ing hyperactivation of the STAT3 pathway. Convergence of mechanisms from con- comitant high expression of both IL6R and ADAR1 culminates in enhanced sur- vival of MM cell survival, potentially contributing to the poor disease prognosis in patients with 1q21 amplification.
by being its downstream transcriptional target and its upstream mediator. This feed-forward regulatory loop causes an augmented oncogenic effect, with both STAT3- induced P150 expression and P150-induced STAT3 protein stabilization acting in concert to promote manifestation of an aggressive disease. Coupled with the overexpression of IL6R that drives the hypersensitivity of the STAT3 path- way, it is plausible that these multi-oncogenic hits could contribute to the poor prognosis in the 1q21(amp) patients.
Although IL6R has been documented to be a predictor of poor outcome in myeloma patients,5,21,22,41 there have not been proper biological studies associating it to myeloma- genicity. Our notion of high IL6R acting as an ever-ready receptor for the IL6 ligand was proven when 1q21(amp) cells which constitutively express endogenous IL6R showed hastened and sustainable STAT3 pathway activa- tion and enhanced IL6-induced-growth. This suggests that
1402
haematologica | 2020; 105(5)