Page 25 - Haematologica April 2020
P. 25
MDS PDX: from no options to many
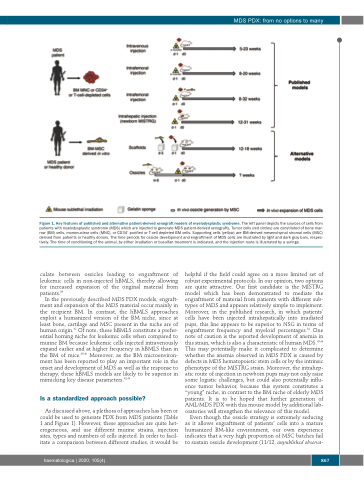
Figure 1. Key features of published and alternative patient-derived xenograft models of myelodysplastic syndrome. The left panel depicts the sources of cells from patients with myelodysplastic syndrome (MDS) which are injected to generate MDS patient-derived xenografts. Tumor cells (red circles) are constituted of bone mar- row (BM) cells, mononuclear cells (MNC), or CD34+ purified or T-cell depleted BM cells. Supporting cells (yellow) are BM-derived mesenchymal stromal cells (MSC) derived from patients or healthy donors. The time periods for ossicle development and engraftment of MDS cells are illustrated by light and dark gray bars, respec- tively. The time of conditioning of the animal, by either irradiation or busulfan treatment is indicated, and the injection route is illustrated by a syringe.
culate between ossicles leading to engraftment of leukemic cells in non-injected hBMLS, thereby allowing for increased expansion of the original material from patients.39
In the previously described MDS PDX models, engraft- ment and expansion of the MDS material occur mainly in the recipient BM. In contrast, the hBMLS approaches exploit a humanized version of the BM niche, since at least bone, cartilage and MSC present in the niche are of human origin.42 Of note, these hBMLS constitute a prefer- ential homing niche for leukemic cells when compared to murine BM because leukemic cells injected intravenously expand earlier and at higher frequency in hBMLS than in the BM of mice.39,40 Moreover, as the BM microenviron- ment has been reported to play an important role in the onset and development of MDS as well as the response to therapy, these hBMLS models are likely to be superior in mimicking key disease parameters.43,44
Is a standardized approach possible?
As discussed above, a plethora of approaches has been or could be used to generate PDX from MDS patients (Table 1 and Figure 1). However, these approaches are quite het- erogeneous, and use different murine strains, injection sites, types and numbers of cells injected. In order to facil- itate a comparison between different studies, it would be
helpful if the field could agree on a more limited set of robust experimental protocols. In our opinion, two options are quite attractive. Our first candidate is the MISTRG model which has been demonstrated to mediate the engraftment of material from patients with different sub- types of MDS and appears relatively simple to implement. Moreover, in the published research, in which patients’ cells have been injected intrahepatically into irradiated pups, this line appears to be superior to NSG in terms of engraftment frequency and myeloid percentages.34 One note of caution is the reported development of anemia in this strain, which is also a characteristic of human MDS.45,46 This may potentially make it complicated to determine whether the anemia observed in MDS PDX is caused by defects in MDS hematopoietic stem cells or by the intrinsic phenotype of the MISTRG strain. Moreover, the intrahep- atic route of injection in newborn pups may not only raise some logistic challenges, but could also potentially influ- ence tumor behavior, because this system constitutes a “young” niche, in contrast to the BM niche of elderly MDS patients. It is to be hoped that further generation of AML/MDS PDX with this mouse model by additional lab- oratories will strengthen the relevance of this model.
Even though the ossicle strategy is extremely seducing as it allows engraftment of patients’ cells into a mature humanized BM-like environment, our own experience indicates that a very high proportion of MSC batches fail to sustain ossicle development (11/12, unpublished observa-
haematologica | 2020; 105(4)
867