Page 23 - Haematologica April 2020
P. 23
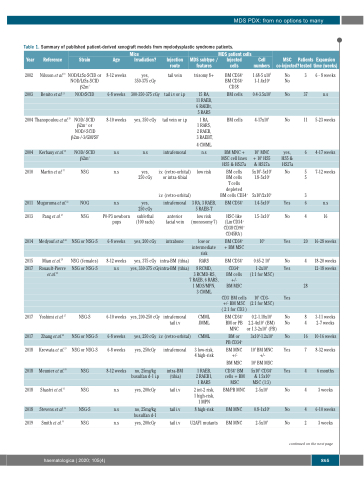
Table 1. Summary of published patient-derived xenograft models from myelodysplastic syndrome patients.
MDS PDX: from no options to many
Year
2002
2003
Reference Strain
Nilsson et al.18 NOD/LtSz-SCID or NOD/LtSz-SCID
Mice Irradiation?
yes, 350-375 cGy
300-350-375 cGy
yes, 350 cGy
n.s
yes, 250 cGy
yes,
250 cGy
sublethal (100 rads)
yes, 200 cGy
yes, 375 cGy
MDS patient cells
Age
8-12 weeks
6-8 weeks
8-10 weeks
n.s
n.s
n.s
P0-P3 newborn pups
6-8 weeks
8-12 weeks
n.s
6-10 weeks
6-8 weeks
6-8 weeks
8-12 weeks
n.s
n.s
n.s
Injection route
tail vein
tail i.v or i.p
tail vein or i.p
intrafemoral
MDS subtype / features
trisomy 8+
15 RA, 11 RAEB, 6 RAEBt, 5 RARS
1 RA,
1 RARS, 2 RAEB, 3 RAEBT,
4 CMML
n.s
low risk
3 RA, 3 RAEB,
5 RAEB-T
low risk (monosomy 7)
low or intermediate risk
RARS
8 RCMD,
3 RCMD-RS, 7 RAEB, 6 RARS,
1 MDS/MPN, 3 CMML
CMML JMML
CMML
3 low-risk, 4 high-risk
1 RAEB, 2 RAEB1, 1 RARS
2 int-2 risk, 1 high-risk, 1 MPN
8 high-risk
U2AF1 mutants
Injected cells
BM CD34+ BM CD34+ CD38-
BM cells
BM cells
BM MNC + MSC cell lines HS5 & HS27a
BM cells BM cells
T cells depleted BM cells CD34+
BM CD34+
HSC-like (Lin-CD34+ CD38-CD90+ CD45RA-)
BM CD34+ + BM MSC
BM CD34+
CD34+ BM cells +/- BM MSC
CD3- BM cells +/- BM MSC ( 2:1 for CD3-)
Cell numbers
1.68-5 x105 1-1.4x104
0.4-3.5x107
4-17x106
107 MNC + 105 HS5 & HS27a
5x105-5x106 1.8-5x106
5x105/2x106
1.4-5x105
1.5-3x103
105
0.65-2 105
1-2x105 (1:1 for MSC)
106 CD3- (2:1 for MSC)
MSC Patients Expansion co-injected?tested time (weeks)
Benito et al.21
β2m-/- NOD/SCID
NOD/-SCID β2m-/- or
NOD/-SCID β2m-/-3/GM/SF
NOD/-SCID β2m-/-
NSG
NOG
NSG
NSG or NSG-S
NSG (females)
NSG or NSG-S
NSG-S
NSG or NSG-S
NSG or NSG-S
NSG
NSG
NSG-S
NSG
No 3 No
No 37
No 11
yes, 6 HS5 &
HS27a
No 5 5
3
Yes 6
No 4
Yes 20
No 4
Yes
Yes
6-8weeks
n.s
5-23 weeks
4-17 weeks
7-12 weeks
n.s
16
16-28 weeks
18-20 weeks
12-18 weeks
3-11 weeks 2-7 weeks
10-16 weeks
8-32 weeks
6 months
3 weeks
6-10 weeks
3 weeks
2004 Thanopoulou et al.20
2004
2010
2011
2013
2014
2015
2017
2017
2017
2018
2018
2018
2018
2019
Kerbauy et al.19
Martin et al.37
Muguruma et al.22 Pang et al.23
Medyouf et al.24
Mian et al.25 Rouault-Pierre
et al.26
Yoshimi et al.27 Zhang et al.28
Krevvata et al.29 Meunier et al.30
Shastri et al.31 Stevens et al.32
Smith et al.33
i.v. (retro-orbital) or intra-tibial
i.v. (retro-orbital)
intrafemoral
anterior facial vein
intrabone
intra-BM (tibia)
yes, 330-375 cGy intra-BM (tibia)
28
yes, 200-250 cGy
intrafemoral tail i.v
BM CD34+ BM or PB MNC
BM or
PB CD34+
BM MNC +/-
BM MSC
CD34+ BM cells + BM MSC
BM/PB MNC
BM MNC
BM MNC
0.2-1.18x106 2.2-4x106 (BM) or 1.3-2x106 (PB)
3x104-1.2x106
106 BM MNC +/-
105 BM MSC
5x105 CD34+ & 1.5x106 MSC (1:3)
2-5x106
0.8-1x106
2-5x106
No 8 No 4
No 16
Yes 7
Yes 4
No 4
No 4
No 2
yes, 250 cGy i.v. (retro-orbital)
yes, 250cGy intrafemoral
no, 25mg/kg intra-BM busulfan d-1 i.p (tibia)
yes, 200cGy tail i.v
no, 25mg/kg tail i.v
busulfan d-1
yes, 200cGy tail i.v
continued on the next page
haematologica | 2020; 105(4)
865