Page 55 - 2019_12-Haematologica-web
P. 55
Disruption of MBD2-NuRD induces high HbF levels
mutation.42 However, significant technological and safety barriers remain, and the vast majority of the worldwide sickle cell burden lies in underdeveloped nations, where small molecule therapeutics will likely be more feasible than cell-based therapy in the foreseeable future. We have pursued a structure-function guided approach to identify heretofore “undruggable” small molecule targets for dis- ruption of the MBD2-NuRD gene silencing effects.26 We show that enforced expression of mutant MBD2- CCmutsgR (D366R/R375E/R380E) fails to suppress g-glo- bin in MBD2KO HUDEP-2 cells while expression of WT MBD2 partially rescues the MBD2KO phenotype consis- tent with published data showing that enforced expres- sion of the CC domain of GATAD2A competitively dis- rupts the MBD2-GATAD2A interaction and mimics the effect of MBD2 Kd in murine CID cells bearing a human β-YAC.27
We previously identified two critical residues in the intrinsically disordered region of MBD2 that are necessary and sufficient for mediating recruitment of the HDAC core complex (HDCC) and silencing of a methylated tumor suppressor gene in breast cancer cells.25 Enforced expression of MBD2-IDRmutsgR (R286E/L287A) also fails to suppress g-globin in MBD2KO HUDEP-2 cells. Moreover, this mutation disrupts the inherent helical propensity of the unstructured domain. While this obser- vation does not exclude the possibility that R286 or L287
directly interact with components of the HDCC, it indi- cates that these two residues contribute to the structural propensity of the IDR. The inherent structural propensity of intrinsically disordered regions can be critical for their high-affinity association with binding partners.43-45 Hence, our results support a model in which the helical propensi- ty of the IDR is necessary for binding to the HDCC (Figure 5C), raising the possibility that inhibiting this structural propensity with a small molecule ligand could disrupt for- mation of a functional NuRD complex. To our knowledge, these results show for the first time the functional effects of disrupting small protein interaction domains within the MBD2-NuRD complex on g-globin gene silencing in human erythroid cells. We infer from this that small mol- ecules or peptides which specifically bind to and disrupt the IDR or CC domains of MBD2 respectively would be potential candidates for the treatment of the β-hemoglo- binopathies. Intrinsically disordered regions have recently been implicated as potential drug targets and novel screen- ing strategies have been utilized to target them.46-48
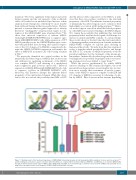
While the precise mechanism(s) by which MBD2 enforces silencing of HbF remains incomplete, the work presented here demonstrates several insights into its func- tion, most crucially that recruitment of specific compo- nents of the NuRD co-repressor complex via the IDR and CC domains of MBD2 is necessary for silencing of g-glo- bin by MBD2. One observation that remains perplexing
Figure 7. Working model of the functional importance of MBD2-NuRD interacting domains in fetal hemoglobin (HbF) regulation. Previous work by our group9,25,27 and the findings presented here support a model in which the HDAC core complex (HDCC) members HDAC1/2, RBBP4-7, and MTA1/2 are recruited to MBD2-NuRD through an intrinsically disordered region of MBD2, while GATAD2A/B and CHD4 are recruited through a c-terminal coiled-coil motif of MBD2; independently decou- pling either subcomplex results in an abrogation of MBD2-NuRD-mediated HbF silencing.
haematologica | 2019; 104(12)
2369