Page 198 - 2019_10 resto del Mondo_web
P. 198
E. Karampini et al.
Ex vivo HPS-2 endothelial cells have impaired stimu- lus-induced Weibel-Palade body exocytosis
To investigate the contribution of AP-3 dependent matu- ration on the degranulation efficiency of WPB in endothe- lial cells, we measured the stimulus-induced vWF release in HPS-2 BOEC. We observed that, under unstimulated con- ditions, the release of vWF is unaffected in the HPS-2 BOEC compared to WT (Figure 3A). However, upon stim- ulation, HPS-2 BOEC showed a clear defect in vWF release with both Ca2+ (histamine) and cAMP-mediated (forskolin) secretagogues (Figure 3B). As an alternative
measurement of WPB exocytosis, we also investigated how other WPB proteins respond to histamine treatment. We stimulated WT and HPS-2 BOEC and measured CD62P exposure on the plasma membrane (Figure 3D). We found that significantly less CD62P was expressed on HPS- 2 BOEC plasma membrane when compared to healthy control BOEC upon histamine stimulation. The reduction in the stimulus-induced surface expression of CD62P and release of vWF in HPS-2 BOEC suggests that defects in the AP-3 dependent maturation of WPB alter the exocytotic potential of these organelles in endothelial cells.
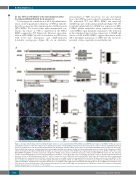
AB
CD
EF
Figure 1. Disrupted trafficking of CD63 to Weibel-Palade bodies in ex vivo patient-derived HPS-2 BOEC and CRISPR/Cas9-engineered AP3B1-/- BOEC. (A) Cartoon depicting the mutations of the HPS-2 patient in the AP3B1 gene and the corresponding predicted truncated protein products relative to the full length AP-3β1 domain structure. (B) Expression of AP-3β1 in WT and HPS-2 BOEC. (B) Healthy WT and HPS-2 BOEC lysates were separated with SDS-PAGE and were immunoblotted for AP- 3β1; α-tubulin was used as a loading control. Molecular weight standards are indicated on the left (kDa) and quantification of relative AP-3β1 expression in HPS- 2 BOEC normalized to AP-3β1 in healthy WT BOEC (right). (C) Cartoon representation of the CRISPR\Cas9-engineering strategy to generate AP3B1-/- BOEC lines. Guide RNA (gRNA4 and gRNA5) are shown underneath a fragment of AP3B1 exon 1 sequence with their protospacer adjacent motif (PAM) indicated in red. The mutations and the corresponding predicted truncated protein products of 2 AP3B1-/- clones (4.F6 and 5.A10, respectively) are shown relative to the full length AP-3β1 domain structure. (D) Loss of AP-3β1 expression in AP3B1-/- BOEC. (D) Lysates of clonal CTRL BOEC and 2 clonal AP3B1-/- BOEC lines (4.F6 and 5.A10) were separated with SDS-PAGE and were immunoblotted for AP-3β1; α-tubulin was used as a loading control (left) and quantification of relative AP-3β1 expression in clonal AP3B1-/- BOEC lines normalized to AP-3β1 in clonal CTRL BOEC (right). (E-F) Immunostaining of BOEC for vWF (magenta) and CD63 (cyan) in WT versus HPS-2 BOEC (E left) and CTRL versus AP3B1-/- 4.F6 BOEC (F), respectively. Boxed areas are magnified in the right part. Yellow arrowheads indicate the position of WPB in both channels. Scale bars represent 10 μm. (E) Proportion of CD63 immunoreactivity that is found on WPB (right, top) and proportion of WPB that contain CD63 immunoreactivity (right, bottom). Student t-test, ***P<0.001, ****P<0.0001.
2094
haematologica | 2019; 104(10)