Page 198 - 2019_07 resto del Mondo-web
P. 198
L. Bury et al.
reticulated platelet percentages (16.6% and 23.6%) corre- sponded to periods in which the patient was pregnant. Her bone marrow biopsy showed an increased number of megakaryocytes (Online Supplementary Figure S1).
von Willebrand factor binding to megakaryocytes
von Willebrand factor bound to the surface of human PT-vWD megakaryocytes, co-localizing with GPIba, was detected by confocal microscopy (Figure 1A) and flow cytometry (Figure 1B) on days 7, 10 and 14 of cell differ- entiation, while control megakaryocytes did not show bound vWF at any of the differentiation times. vWF did not co-localize with GPIba intracellularly (Online Supplementary Figure S2). vWF was secreted from megakaryocytes starting from day 7 until day 14 of differ- entiation (Online Supplementary Figure S3A).
von Willebrand factor was not detected on the surface of megakaryocytes from TgG233V mice (Online Supplementary Figure S3B), in line with previous observa- tions.19 It cannot be excluded that vWF is bound to murine mutant megakaryocytes in vivo but that the quantity is too low to be detected by immunofluorescence, or that vWF bound to the megakaryocyte surface is lost during murine
megakaryocyte isolation, culture and analysis. However, megakaryocytes from TgG233V mice bound vWF after stim- ulation with a lower dose of ristocetin compared to megakaryocytes from control mice, confirming increased affinity for vWF (Figure 1C).
Megakaryocyte differentiation, spreading, proplatelet formation and migration
The percentage of peripheral-blood derived CD45+/CD34+ cells differentiating in megakaryocytes was comparable in PT-vWD and controls (PT-vWD 37.3±7.2%, controls 41.2±9.3%), with a slightly but sig- nificantly lower percentage of PT-vWD megakaryocytes reaching stage IV of differentiation (Figure 2A). Transmission electron microscopy of PT-vWD megakary- ocytes did not show ultrastructural abnormalities (Online Supplementary Figure S4).
The fraction of megakaryocytes spreading on immobi- lized fibrinogen, vWF or type I collagen was similar in PT- vWD and controls (Online Supplementary Figure S5A and B). The fraction of megakaryocytes generating proplatelets, either in suspension or on fibrinogen- or VWF-coated cov- erslips, was also comparable in PT-vWD and controls
A
B
CEF
D
G
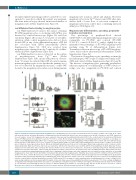
Figure 2. Proplatelet formation from human megakaryocytes. (A) Megakaryocyte percentage and maturation. The percentage of CD41+ cells at day 14 of culture was measured by flow cytometry. Maturation of megakaryocytes was determined by fluorescence microscopy based on ploidy, cell diameter, and CD41 expression. For each sample, at least 100 megakaryocytes were evaluated. Data represent mean±Standard Deviation (SD) for five controls and for the platelet-von Willebrand disease (PT-vWD) patient on five different occasions; *P<0.05 vs. control. (B) Percentage of megakaryocytes-extending proplatelets in suspension, or onto glass cov- erslips coated with fibrinogen (FBG), vWF or type I collagen (Coll I). Data represent mean±Standard Error of Mean (SEM) of five controls and of five different prepa- rations from the PT-vWD patient (*P<0.05 vs. controls). (C) Representative images of control and PT-vWD megakaryocytes plated on type I collagen. Scale bars=20 μm. β1 tubulin is stained green (Alexa Fluor® 488 Goat Anti-Rabbit IgG; Molecular Probes, Life Technologies, Milan, Italy), polymerized actin is stained red (rho- damine-phalloidine; Molecular Probes), and nuclei are stained blue with Hoechst. Specimens were mounted with the ProLong Antifade medium (Molecular Probes), analyzed at room temperature with a Carl Zeiss Axio Observer.A1 fluorescence microscope (Carl Zeiss Inc., Oberkochen, Germany) using a 63x/1.4 Plan-Apochromat oil-immersion objective and images acquired using the AxioVision software (Carl Zeiss Inc.). All polynucleated cells extending protrusions with terminal tips were defined as proplatelet-forming megakaryocytes while those displaying a flattened shape with actin organized into focal adhesion points and fibers as spreading megakaryocytes. Scale bars=20 μm. (D) Number of proplatelet tips generated by megakaryocytes. Individual data, means and 95% Confidence Interval (95%CI) are shown (*P<0.05 vs. control). Measures were carried out on megakaryocytes from five controls and five different preparations from the PT-vWD patient. (E) Diameter of proplatelet tips generated by megakaryocytes. Individual data, means and 95%CI are shown (*P<0.05 vs. control). Measures were carried out on megakaryocytes from five controls and five different preparations from the PT-vWD patient. (F) Percentage of control megakaryocytes-extending proplatelets on type I collagen (Coll I) under resting conditions or after incubation with 1.5 mg/mL of ristocetin. Data represent mean±SEM of five different experiments (*P<0.05 vs. resting). (G) Migration of megakaryocytes through transwell filters uncoated or coated with type I collagen in response to SDF-1a (100 ng/mL). Chemotaxis index (CI) expresses the number of cells that have passed through the filter in response to SDF-1α divided by the number of cells passed in the absence of SDF-1a (n=4; *P<0.05 vs. control). Measures were carried out on megakaryocytes from four controls and four different preparations from the PT-vWD patient.
1476
haematologica | 2019; 104(7)