Page 25 - 2018_12-Haematologica-web
P. 25
Role of osteogenic niche in AML progression
or bone progenitor cells, but not more differentiated osteoblasts. These data are consistent with our observa- tions that AML-MSCs show characteristics of osteoprog- enitors but not of mature osteoblasts. AML-MSCs express early-stage osteoblast markers, including osterix, RUNX2, and Col1a1, but not mature osteoblast markers such as osteocalcin.50 In addition, functional assays revealed that AML-MSCs stained positive for ALP enzyme activity but were negative for alizarin red S stain- ing.50 These observations suggest that AML-MSCs can differentiate into committed osteoprogenitors, but not mature osteoblasts. These data were also validated by co- culture of AML cell lines with normal BM-MSCs in vitro and by different AML mouse models.50,63 These findings
also do not contradict the observations of Frisch et al.,56 Geyh et al.64 and Krevvata et al.65 since the osteoblasts inhibited in these studies were marked by osteocalcin. Using intravital microscopy, Duarte et al. also showed a significant depletion of Col2.3 promoter-expressing mature osteoblasts in areas with a high level of AML fil- tration.66 Collectively, stalling the maturation of osteoblast precursors appears to be a key step in AML ini- tiation and progression (Figure 2).
This differentiation blockade could be mediated by dif- ferent AML-derived factors. Kumar et al.63 reported upreg- ulation of DKK1, a negative regulator of osteogenesis, when co-culturing AML-derived exosomes with BM MSCs. Of particular interest, a short-term dose of DKK1
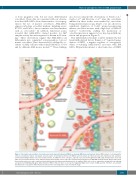
Figure 2. Schematic representation of normal versus acute myeloid leukemia (AML)-bone marrow (BM) microenvironment. Normal BM consists of osteoprogenitor cells, pre-osteoblasts, mature osteoblasts, and osteocytes, mesenchymal stromal cells (MSCs) and osteoclasts at endosteal niche and endothelial cells, pericytes, and non-myelinating Schwann cell at non-endosteal niche. In addition to these cell types, adipocytes are present throughout the BM cavity. Hematopoietic stem cells (HSC) are present in both niche areas and gain support from stromal cells to stay quiescent and self-renew, whereas in AML BM, leukemic blasts displace HSCs from the protective niche area and occupy this sanctuary, thereby affecting normal hematopoiesis. In addition, AML cells create or expand the existing niche by inducing osteogenic but inhibiting adipogenic differentiation in MSCs. However, there are no reports suggesting higher bone volume in AML patients. Therefore, it is possible that induction of osteogenic differentiation is halted at the osteo-progenitor or pre-osteoblastic stage.
haematologica | 2018; 103(12)
1949