Page 26 - 2018_12-Haematologica-web
P. 26
P.M. Le et al.
inhibitor promoted terminal differentiation of these osteo- lineage-primed MSCs in vitro and improved survival of mice engrafted with AML. This suggests a tight coupling of AML development with impaired maturation of osteo- progenitors. The fact that disruption of miRNA processing in immature, but not mature, osteoblasts could trigger AML development35 implies that deregulation of the matu- ration process at the post-transcriptional level might play a role in its failure. It would be interesting to further investi- gate the function of the miR-29 family, whose members are commonly down-regulated in AML blasts67 while engaging Wnt signaling antagonists, such as DKK1, in a feedback loop to promote osteolineage development.68 Another potential mediator is IL-1β, a pleiotropic cytokine produced abundantly by AML blasts69 that has been shown
to suppress osteogenesis of MSCs in periodontal tissue at a high physiological level.70 Whether one or more pathways are involved in causing this lack of osteoblast maturation remains to be elucidated.
Intriguingly, the effect of maturing osteoblasts on leukemia progression may be context-dependent and dis- ease-specific. Schepers et al.71 showed that development of CML-like MPN induced by the BCR/ABL oncogene led to the expansion of a mixture of immature and mature osteoblasts that formed BM fibrosis and had decreased capacity to support HSCs. Krevvata et al.65 reported a strong correlation between the decrease in mature osteoblasts in mice and aggravated engraftment of differ- ent acute leukemia cell lines. In this study, over-stimulat- ing Osc+ osteoblast production by inhibiting gut-derived
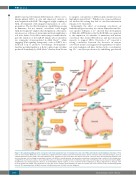
Figure 3. Key signaling pathways in the osteogenic niche that regulate the fate of hematopoietic stem cells (HSC) and leukemia stem/initiating cells (LSCs). HSCs home to bone marrow (BM) and receive maintenance signals from both endosteal and non-endosteal niches. Various cell types in the perisinusoid region, such as CXCL12-abundant reticular (CAR) cells and endothelial cells, maintain less quiescent HSCs via CXCL12 and stem cell factor (SCF). Non-myelinating Schwann cells and osteolineage cells, including mesenchymal stromal cells (MSCs), osteoprogenitors, and premature and mature osteoblasts, play a major role in retaining slow- cycling HSCs near the bone surface. LSCs exploit the same cues from the osteogenic niche to hibernate and evade chemotherapy. In acute myeloid leukemia (AML), the BM niches are relatively hypoxic, whereas, in normal BM the hypoxic regions are more restricted to HSC-residing areas. Ang-1: angiopoietin 1; CXCL12: C-X-C motif chemokine 12; CXCR4: C-X-C chemokine receptor type 4; LSC: leukemic stem/initiating cell; OPN: osteopontin; SCF: stem cell factor; TGF-β: transforming growth factor β; TPO: thrombopoietin; VCAM-1: vascular cell adhesion protein 1; VLA-4: very late antigen-4.
1950
haematologica | 2018; 103(12)