Page 234 - Haematologica Vol. 107 - September 2022
P. 234
LETTER TO THE EDITOR
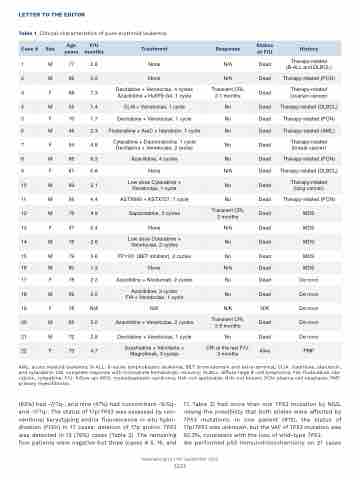
Table 1. Clinical characteristics of pure erythroid leukemia.
Case #
Sex
Age years
F/U months
Treatment
Response
Status at F/U
History
1
M
77
2.8
None
N/A
Dead
Therapy-related (B-ALL and DLBCL)
2
M
66
0.2
None
N/A
Dead
Therapy-related (PCN)
3
F
68
7.3
Decitabine + Venotoclax, 4 cycles Azacitidine + Hu5F9-G4, 1 cycle
Transient CRi, 2.1 months
Dead
Therapy-related (ovarian cancer)
4
M
55
1.4
CLIA + Venetoclax, 1 cycle
No
Dead
Therapy-related (DLBCL)
5
F
70
1.7
Decitabine + Venetoclax, 1 cycle
No
Dead
Therapy-related (PCN)
6
M
48
2.3
Fludarabine + AraC + Idarubicin, 1 cycle
No
Dead
Therapy-related (AML)
7
F
54
4.8
Cytarabine + Daunorubicine, 1 cycle Decitabine + Venetoclax, 2 cycles
No
Dead
Therapy-related (breast cancer)
8
M
66
6.3
Azacitidine, 4 cycles
No
Dead
Therapy-related (PCN)
9
F
81
0.8
None
N/A
Dead
Therapy-related (DLBCL)
10
M
69
2.1
Low dose Cytarabine + Venetoclax, 1 cycle
No
Dead
Therapy-related (lung cancer)
11
M
56
4.4
ASTX660 + ASTX727, 1 cycle
No
Dead
Therapy-related (PCN)
12
M
76
4.9
Sapacitabine, 3 cycles
Transient CRi, 2 months
Dead
MDS
13
F
37
0.4
None
N/A
Dead
MDS
14
M
78
2.6
Low dose Cytarabine + Venetoclax, 2 cycles
No
Dead
MDS
15
M
79
3.6
FF1101 (BET inhibitor), 2 cycles
No
Dead
MDS
16
M
60
1.3
None
N/A
Dead
MDS
17
F
78
2.2
Azacitidine + Nivolumab, 2 cycles
No
Dead
De novo
18
M
59
5.5
Azacitidine, 3 cycles FIA + Venetoclax, 1 cycle
No
Dead
De novo
19
F
78
N/A
N/K
N/K
N/K
De novo
20
M
65
5.0
Azacitidine + Venetoclax, 2 cycles
Transient CRi, 2.6 months
Dead
De novo
21
M
72
2.8
Decitabine + Venetoclax, 1 cycle
No
Dead
De novo
22
F
73
4.7
Azacitadine + Venclexta + Magrolimab, 3 cycles
CRi at the last F/U, 3 months
Alive
PMF
AML: acute myeloid leukemia; B-ALL: B-acute lymphoblastic leukemia; BET: bromodomain and extra-terminal; CLIA: cladribine, idarubicin, and cytarabine; CRi: complete response with incomplete hematologic recovery; DLBCL: diffuse large B-cell lymphoma; FIA: fludarabine, ida- rubicin, cytarabine; F/U: follow up; MDS: myelodysplastic syndrome; N/A: not applicable; N/K: not known; PCN: plasma cell neoplasm; PMF: primary myelofibrosis.
(63%) had -7/7q-, and nine (47%) had concomitant -5/5q- and -7/7q-. The status of 17p/TP53 was assessed by con- ventional karyotyping and/or fluorescence in situ hybri- dization (FISH) in 17 cases: deletion of 17p and/or TP53 was detected in 13 (76%) cases (Table 2). The remaining four patients were negative but three (cases # 5, 15, and
17, Table 2) had more than one TP53 mutation by NGS, raising the possibility that both alleles were affected by TP53 mutations. In one patient (#12), the status of 17p/TP53 was unknown, but the VAF of TP53 mutation was 92.3%, consistent with the loss of wild-type TP53.
We performed p53 immunohistochemistry on 21 cases
Haematologica | 107 September 2022
2233