Page 115 - Haematologica Vol. 107 - September 2022
P. 115
ARTICLE - iLLUMINATE: final analysis C. Moreno et al.
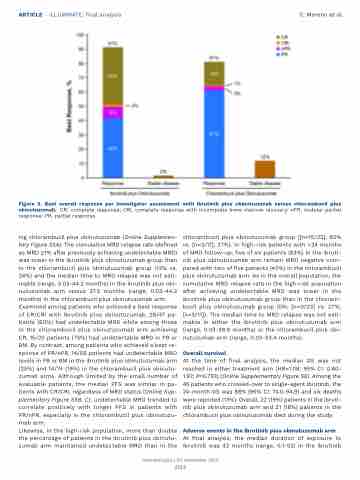
Figure 5. Best overall response per investigator assessment with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab. CR: complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; PR, partial response.
ing chlorambucil plus obinutuzumab (Online Supplemen- tary Figure S5A). The cumulative MRD relapse rate (defined as MRD ≥1% after previously achieving undetectable MRD) was lower in the ibrutinib plus obinutuzumab group than in the chlorambucil plus obinutuzumab group (12% vs. 24%) and the median time to MRD relapse was not esti- mable (range, 0.03-44.2 months) in the ibrutinib plus obi- nutuzumab arm versus 37.5 months (range, 0.03-44.2 months) in the chlorambucil plus obinutuzumab arm. Examined among patients who achieved a best response of CR/CRi with ibrutinib plus obinutuzumab, 28/47 pa- tients (60%) had undetectable MRD while among those in the chlorambucil plus obinutuzumab arm achieving CR, 15/20 patients (75%) had undetectable MRD in PB or BM. By contrast, among patients who achieved a best re- sponse of PR/nPR, 14/56 patients had undetectable MRD levels in PB or BM in the ibrutinib plus obinutuzumab arm (25%) and 14/74 (19%) in the chlorambucil plus obinutu- zumab arms. Although limited by the small number of evaluable patients, the median PFS was similar in pa- tients with CR/CRi, regardless of MRD status (Online Sup- plementary Figure S5B, C); undetectable MRD trended to correlate positively with longer PFS in patients with PR/nPR, especially in the chlorambucil plus obinutuzu- mab arm.
Likewise, in the high-risk population, more than double the percentage of patients in the ibrutinib plus obinutu- zumab arm maintained undetectable MRD than in the
chlorambucil plus obinutuzumab group ([n=15/23]; 65% vs. [n=3/11]; 27%). In high-risk patients with >24 months of MRD follow-up, five of six patients (83%) in the ibruti- nib plus obinutuzumab arm remain MRD negative com- pared with two of five patients (40%) in the chlorambucil plus obinutuzumab arm. As in the overall population, the cumulative MRD relapse rate in the high-risk population after achieving undetectable MRD was lower in the ibrutinib plus obinutuzumab group than in the chloram- bucil plus obinutuzumab group (0%; [n=0/23] vs. 27%; [n=3/11]). The median time to MRD relapse was not esti- mable in either the ibrutinib plus obinutuzumab arm (range, 0.03-38.6 months) or the chlorambucil plus obi- nutuzumab arm (range, 0.03-33.4 months).
Overall survival
At the time of final analysis, the median OS was not reached in either treatment arm (HR=1.08; 95% CI: 0.60- 1.97; P=0.793) (Online Supplementary Figure S6). Among the 45 patients who crossed-over to single-agent ibrutinib, the 24-month OS was 88% (95% CI: 74.0-94.9) and six deaths were reported (13%). Overall, 22 (19%) patients in the ibruti- nib plus obinutuzumab arm and 21 (18%) patients in the chlorambucil plus obinutuzumab died during the study.
Adverse events in the ibrutinib plus obinutuzumab arm
At final analysis, the median duration of exposure to ibrutinib was 42 months (range, 0.1-52) in the ibrutinib
Haematologica | 107 September 2022
2114