Page 116 - Haematologica Vol. 107 - September 2022
P. 116
ARTICLE - iLLUMINATE: final analysis C. Moreno et al.
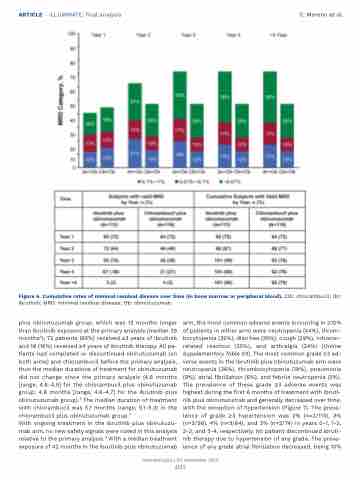
Figure 6. Cumulative rates of minimal residual disease over time (in bone marrow or peripheral blood). Clb: chlorambucil; Ibr: ibrutinib; MRD: minimal residual disease; Ob: obinutuzumab.
plus obinutuzumab group, which was 13 months longer than ibrutinib exposure at the primary analysis (median 29 months3); 73 patients (65%) received ≥3 years of ibrutinib and 18 (16%) received ≥4 years of ibrutinib therapy. All pa- tients had completed or discontinued obinutuzumab (on both arms) and chlorambucil before the primary analysis, thus the median durations of treatment for obinutuzumab did not change since the primary analysis (4.6 months [range, 4.6-4.9] for the chlorambucil plus obinutuzumab group; 4.6 months [range, 4.6-4.7] for the ibrutinib plus obinutuzumab group).3 The median duration of treatment with chlorambucil was 5.1 months (range, 5.1-5.3) in the chlorambucil plus obinutuzumab group.3
With ongoing treatment in the ibrutinib plus obinutuzu- mab arm, no new safety signals were noted in this analysis relative to the primary analysis.3 With a median treatment exposure of 42 months in the ibrutinib plus obinutuzumab
arm, the most common adverse events (occurring in ≥10% of patients in either arm) were neutropenia (44%), throm- bocytopenia (35%), diarrhea (35%), cough (29%), infusion- related reaction (25%), and arthralgia (24%) (Online Supplementary Table S3). The most common grade ≥3 ad- verse events in the ibrutinib plus obinutuzumab arm were neutropenia (36%), thrombocytopenia (19%), pneumonia (9%), atrial fibrillation (6%), and febrile neutropenia (5%). The prevalence of these grade ≥3 adverse events was highest during the first 6 months of treatment with ibruti- nib plus obinutuzumab and generally decreased over time, with the exception of hypertension (Figure 7). The preva- lence of grade ≥3 hypertension was 2% (n=2/113), 3% (n=3/98), 4% (n=3/84), and 3% (n=2/74) in years 0-1, 1-2, 2-3, and 3-4, respectively. No patient discontinued ibruti- nib therapy due to hypertension of any grade. The preva- lence of any grade atrial fibrillation decreased, being 10%
Haematologica | 107 September 2022
2115