Page 113 - Haematologica Vol. 107 - September 2022
P. 113
ARTICLE - iLLUMINATE: final analysis
C. Moreno et al.
tuzumab arm (70% vs. 12%). At 48 months, the PFS benefit was maintained since the time of the primary analysis at 30 months (70% vs. 77%, respectively) (Figure 2). The ex- clusion of patients with del(17p) did not affect 42-month PFS estimates for the rest of the high-risk population, which were 71% and 14%, respectively, for the two treat- ment arms (Online Supplementary Figure S2).
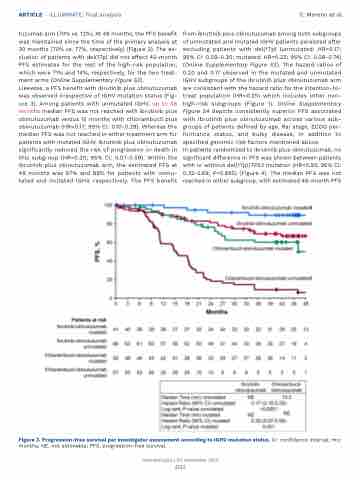
Likewise, a PFS benefit with ibrutinib plus obinutuzumab was observed irrespective of IGHV mutation status (Fig- ure 3). Among patients with unmutated IGHV, up to 48 months median PFS was not reached with ibrutinib plus obinutuzumab versus 15 months with chlorambucil plus obinutuzumab (HR=0.17; 95% CI: 0.10-0.29). Whereas the median PFS was not reached in either treatment arm for patients with mutated IGHV, ibrutinib plus obinutuzumab significantly reduced the risk of progression or death in this subgroup (HR=0.20; 95% CI: 0.07-0.59). Within the ibrutinib plus obinutuzumab arm, the estimated PFS at 48 months was 67% and 89% for patients with unmu- tated and mutated IGHV, respectively. The PFS benefit
from ibrutinib plus obinutuzumab among both subgroups of unmutated and mutated IGHV patients persisted after excluding patients with del(17p) (unmutated: HR=0.17; 95% CI: 0.09-0.30; mutated: HR=0.25; 95% CI: 0.08-0.74) (Online Supplementary Figure S3). The hazard ratios of 0.20 and 0.17 observed in the mutated and unmutated IGHV subgroups of the ibrutinib plus obinutuzumab arm are consistent with the hazard ratio for the intention-to- treat population (HR=0.25) which includes other non- high-risk subgroups (Figure 1). Online Supplementary Figure S4 depicts consistently superior PFS associated with ibrutinib plus obinutuzumab across various sub- groups of patients defined by age, Rai stage, ECOG per- formance status, and bulky disease, in addition to specified genomic risk factors mentioned above.
In patients randomized to ibrutinib plus obinutuzumab, no significant difference in PFS was shown between patients with or without del(17p)/TP53 mutation (HR=0.93; 95% CI: 0.32-2.69; P=0.895) (Figure 4). The median PFS was not reached in either subgroup, with estimated 48-month PFS
Figure 3. Progression-free survival per investigator assessment according to IGHV mutation status. CI: confidence interval; mo: months; NE, not estimable; PFS, progression-free survival.
Haematologica | 107 September 2022
2112