Page 111 - Haematologica Vol. 107 - September 2022
P. 111
ARTICLE - iLLUMINATE: final analysis Results
Patient demographics and characteristics
Two hundred twenty-nine patients were randomized to receive ibrutinib plus obinutuzumab (n=113) or chloram- bucil plus obinutuzumab (n=116). Patient baseline demo- graphics and disease characteristics were generally comparable between treatment arms (Online Supplemen- tary Table S1). The median age was 71 years (range, 40-87), with most patients (n=183; 80%) aged ≥65 years. Most pa- tients (148 [65%]) had high-risk disease features of del(17p), TP53 mutation, del(11q), or unmutated IGHV.
Patient disposition and treatment
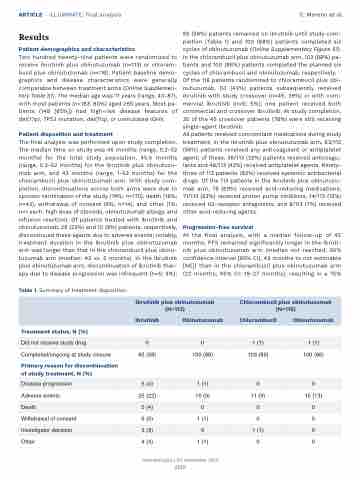
The final analysis was performed upon study completion. The median time on study was 45 months (range, 0.2-52 months) for the total study population, 45.5 months (range, 0.2-52 months) for the ibrutinib plus obinutuzu- mab arm, and 43 months (range, 1-52 months) for the chlorambucil plus obinutuzumab arm. With study com- pletion, discontinuations across both arms were due to sponsor termination of the study (74%; n=170), death (18%; n=42), withdrawal of consent (6%; n=14), and other (1%; n=1 each: high dose of steroids, obinutuzumab allergy, and infusion reaction). Of patients treated with ibrutinib and obinutuzumab, 26 (23%) and 10 (9%) patients, respectively, discontinued these agents due to adverse events; notably, treatment duration in the ibrutinib plus obinutuzumab arm was longer than that in the chlorambucil plus obinu- tuzumab arm (median: 42 vs. 5 months). In the ibrutinib plus obinutuzumab arm, discontinuation of ibrutinib ther- apy due to disease progression was infrequent (n=5; 4%);
C. Moreno et al.
65 (58%) patients remained on ibrutinib until study com- pletion (Table 1) and 100 (88%) patients completed six cycles of obinutuzumab (Online Supplementary Figure S1). In the chlorambucil plus obinutuzumab arm, 103 (89%) pa- tients and 100 (86%) patients completed the planned six cycles of chlorambucil and obinutuzumab, respectively. Of the 116 patients randomized to chlorambucil plus obi- nutuzumab, 50 (43%) patients subsequently received ibrutinib with study crossover (n=45; 39%) or with com- mercial ibrutinib (n=6; 5%); one patient received both commercial and crossover ibrutinib. At study completion, 35 of the 45 crossover patients (78%) were still receiving single-agent ibrutinib.
All patients received concomitant medications during study treatment; in the ibrutinib plus obinutuzumab arm, 63/113 (56%) patients received any anticoagulant or antiplatelet agent; of these, 36/113 (32%) patients received anticoagu- lants and 48/113 (42%) received antiplatelet agents. Ninety- three of 113 patients (82%) received systemic antibacterial drugs. Of the 113 patients in the ibrutinib plus obinutuzu- mab arm, 78 (69%) received acid-reducing medications; 71/113 (63%) received proton pump inhibitors, 14/113 (12%) received H2-receptor antagonists, and 8/113 (7%) received other acid-reducing agents.
Progression-free survival
At the final analysis, with a median follow-up of 45 months, PFS remained significantly longer in the ibruti- nib plus obinutuzumab arm (median not reached; 95% confidence interval [95% CI], 49 months to not estimable [NE]) than in the chlorambucil plus obinutuzumab arm (22 months; 95% CI: 18-27 months), resulting in a 75%
Table 1. Summary of treatment disposition.
Ibrutinib plus obinutuzumab (N=113)
Chlorambucil plus obinutuzumab (N=116)
Ibrutinib
Obinutuzumab
Chlorambucil
Obinutuzumab
Treatment status, N (%)
Did not receive study drug
0
0
1 (1)
1 (1)
Completed/ongoing at study closure
65 (58)
100 (88)
103 (89)
100 (86)
Primary reason for discontinuation of study treatment, N (%)
Disease progression
5 (4)
1 (1)
0
0
Adverse events
25 (22)
10 (9)
11 (9)
15 (13)
Death
5 (4)
0
0
0
Withdrawal of consent
6 (5)
1 (1)
0
0
Investigator decision
3 (3)
0
1 (1)
0
Other
4 (4)
1 (1)
0
0
Haematologica | 107 September 2022
2110