Page 82 - Haematologica May 2022
P. 82
A.M. Evens et al.
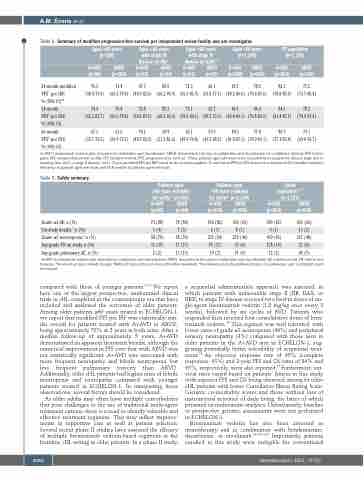
Table 2. Summary of modified progression-free survival per independent review facility and per investigator.
Aged ≥60 years (n=186)
Aged ≥60 years with stage III disease (n=65)*
A+AVD ABVD (n=31) (n=34)
Aged ≥60 years with stage IV disease (n=118)*
A+AVD ABVD (n=51) (n=67)
Aged <60 years (n=1,148)
A+AVD ABVD (n=580) (n=568)
ITT population (n=1,334)
A+AVD ABVD
A+AVD (n=84)
ABVD (n=102)
(n=664)
(n=670)
24-month modified PFS† per IRF,
% (95% CI)20 24-month
PFS‡ per INV, % (95% CI) 60-month PFS‡ per INV, % (95% CI)
70.3
(58.4-79.4) (60.5-79.8) (44.9-82.6) (66.2-90.9) (56.3-81.9) (51.8-77.1) (80.2-86.6) (74.4-81.6) (78.8-85.0) (73.7-80.4)
74.4 70.8 74.8 85.3 74.1 62.7 86.5 80.4 84.5 78.3 (62.2-82.7) (60.6-78.8) (54.2-87.1) (68.2-93.6) (59.6-84.1) (49.5-73.5) (83.4-89.1) (76.8-83.5) (81.4-87.1) (74.9-81.4)
67.1 61.6 70.1 69.9 65.1 57.0 84.3 77.8 80.7 73.1 (55.1-76.5) (50.9-70.7) (48.7-83.9) (51.3-82.6) (49.9-76.8) (43.5-68.5) (81.0-87.1) (74.0-81.1) (77.1-83.8) (69.0-76.7)
71.4
67.7 80.9
71.3 66.1
83.7 78.2
82.1 77.2
A+AVD: brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; CI: confidence interval; INV: investi- gator; IRF: independent review facility; ITT: intention-to-treat; PFS: progression-free survival. *Three patients aged ≥60 years were excluded from analysis by disease stage due to missing data (n=2) or stage II disease (n=1).†2-year modified PFS per IRF based on the primary analysis.‡2- and 5-year PFS per INV based on a median of 60.9 months’extended follow-up in patients aged ≥60 years and 60.8 months in patients aged <60 years.
Table 3. Safety summary.
Grade ≥3 AE, n (%) On-studydeaths,† n(%) Grade ≥3 neutropenia,‡ n (%) Any-grade FN on study, n (%)
Any-grade pulmonary AE, n (%)
Patients aged ≥60 years evaluable for safety* (n=181)
Patients aged <60 years evaluable for safety* (n=1,140)
Safety population*,38 (n=1,321)
A+AVD (n=83)
73 (88) 3(4) 58 (70) 31 (37)
2 (2)
ABVD (n=98)
78 (80) 5(5) 58 (59) 17 (17)
13 (13)
A+AVD (n=579)
476 (82) 6(1) 372 (64) 97 (17)
10 (2)
ABVD (n=561)
356 (63)
8(1)
259 (46)
35 (6)
31 (6)
A+AVD (n=662)
549 (83)
9(1)
430 (65)
128 (19)
12 (2)
ABVD (n=659)
434 (66)
13(2)
317 (48)
52 (8)
44 (7)
A+AVD: brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; AE: adverse events; FN: febrile neu- tropenia.*Received ≥1 dose of study therapy.†Within 30 days of the last dose of frontline treatment.‡Neutropenia includes preferred terms of 'neutropenia' and 'neutrophil count decreased'.
compared with those of younger patients.2-4,6 We report here one of the largest prospective, randomized clinical trials in cHL completed in the contemporary era that have included and analyzed the outcomes of older patients. Among older patients ≥60 years treated in ECHELON-1, we report that modified PFS per IRF was statistically sim- ilar overall for patients treated with A+AVD or ABVD, being approximately 70% at 2 years in both arms. After a median follow-up of approximately 5 years, A+AVD demonstrated an apparent treatment benefit, although the numerical improvement in PFS over that with ABVD was not statistically significant. A+AVD was associated with more frequent neuropathy and febrile neutropenia, but less frequent pulmonary toxicity than ABVD. Additionally, older cHL patients had higher rates of febrile neutropenia and neuropathy compared with younger patients treated in ECHELON-1. In interpreting these observations, several factors should be considered.
As older adults may often have multiple comorbidities that pose challenges to the use of traditional multi-agent treatment options, there is a need to identify tolerable and effective treatment regimens. This may reflect improve- ments in supportive care as well as patient selection. Several recent phase II studies have assessed the efficacy of multiple brentuximab vedotin-based regimens in the frontline cHL setting in older patients. In a phase II study,
a sequential administration approach was assessed, in which patients with unfavorable stage II (IIB, IIAX, or IIBX) to stage IV disease received two lead-in doses of sin- gle-agent brentuximab vedotin (1.8 mg/kg once every 3 weeks), followed by six cycles of AVD. Patients who responded then received four consolidative doses of bren- tuximab vedotin.19 This regimen was well tolerated, with lower rates of grade ≥3 neutropenia (44%) and peripheral sensory neuropathy (4%) compared with those seen in older patients in the A+AVD arm in ECHELON-1, sug- gesting potentially better tolerability of sequential treat- ment.19 An objective response rate of 95% (complete responses: 93%) and 2-year PFS and OS rates of 84% and 93%, respectively, were also reported.19 Furthermore, sur- vival rates varied based on patients’ fitness in this study with superior PFS and OS being observed among fit older cHL patients with lower Cumulative Illness Rating Scale- Geriatric co-morbidity scores and those without loss of instrumental activities of daily living, the latter of which persisted on multivariate analyses. Unfortunately, baseline or prospective geriatric assessments were not performed in ECHELON-1.
Brentuximab vedotin has also been assessed as monotherapy and in combination with bendamustine, dacarbazine, or nivolumab.14,16,24,25 Importantly, patients enrolled in this study were ineligible for conventional
1090
haematologica | 2022; 107(5)