Page 80 - Haematologica May 2022
P. 80
A.M. Evens et al.
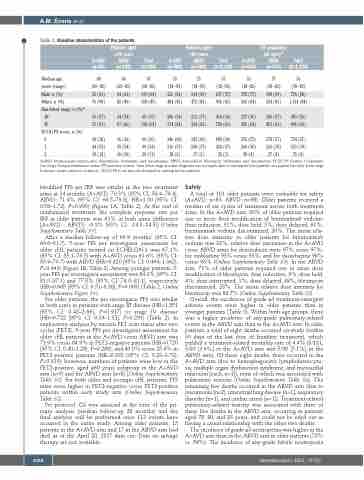
Table 1. Baseline characteristics of the patients. Patients aged
Patients aged
<60 years ABVD (n=568)
ITT population
(all ages)38
ABVD Total
(n=670) (N=1,334)
≥60 years A+AVD ABVD
A+AVD (n=580)
Total (n=1,148)
A+AVD (n=664)
Total (n=84) (n=102) (n=186)
Medianage, 68 66 67 33 33 33 35 37 36
years (range)
Male, n (%)
White, n (%)
Ann Arbor stage, n (%)*
III
IV
ECOG PS score, n (%)†
0
1
2
(60–82) 55 (65) 76 (90)
31 (37) 51 (61)
30 (36)
44 (52)
10 (12)
(60–83) 64 (63) 82 (80)
34 (34) 67 (66)
36 (36)
55 (54)
10 (10)
(60–83) 119 (64) 158 (85)
65 (35) 118 (64)
66 (36)
99 (54)
(18–59) 323 (56) 484 (83)
206 (36) 374 (64)
346 (60) 216 (37)
(18–59) 334 (59) 472 (83)
212 (37) 354 (62)
342 (60) 208 (37)
(18–59) 657 (57) 956 (83)
418 (36) 728 (63)
688 (60) 424 (37)
(18–82) 378 (57) 560 (84)
237 (36) 425 (64)
376 (57) 260 (39)
(18–83) 398 (59) 554 (83)
246 (37) 421 (63)
378 (57) 263 (39)
(18–83)
776 (58) 1,114 (84)
483 (36) 846 (64)
754 (57) 523 (39)
20(11) 18(3) 17(3) 35(3) 28(4) 27(4) 55(4)
A+AVD: brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; ECOG PS: Eastern Cooperative Oncology Group performance status; ITT: intention-to-treat. *Ann Arbor stage at initial diagnosis was not applicable or missing for four patients; one patient had Ann Arbor stage II disease (major protocol violation). †ECOG PS score was not obtained or missing for two patients.
Modified PFS per IRF was similar in the two treatment arms at 24 months (A+AVD: 70.3% [95% CI: 58.4–79.4], ABVD: 71.4% [95% CI: 60.5–79.8], HR=1.00 [95% CI: 0.58–1.72], P=0.993) (Figure 1A, Table 2). At the end of randomized treatment, the complete response rate per IRF in older patients was 61% in both arms (difference [A+AVD - ABVD]: -0.1% [95% CI: -14.5–14.3]) (Online Supplementary Table S1).
After a median follow-up of 60.9 months’ (95% CI: 60.6–61.7), 5-year PFS per investigator assessment for older cHL patients treated on ECHELON-1 was 67.1% (95% CI: 55.1–76.5) with A+AVD versus 61.6% (95% CI: 50.9–70.7) with ABVD (HR=0.820 [95% CI: 0.494–1.362], P=0.443) (Figure 1B; Table 2). Among younger patients, 5- year PFS per investigator assessment was 84.3% (95% CI: 81.0–87.1) and 77.8% (95% CI: 74.0–81.1), respectively (HR=0.665 [95% CI: 0.51–0.88], P=0.003) (Table 2, Online Supplementary Figure S1).
For older patients, the per investigator PFS was similar in both arms in patients with stage III disease (HR=1.051 [95% CI: 0.42–2.66], P=0.917) or stage IV disease (HR=0.722 [95% CI: 0.39–1.33], P=0.291) (Table 2). In exploratory analyses by interim PET scan status after two cycles (PET2), 5-year PFS per investigator assessment for older cHL patients in the A+AVD versus ABVD arm was 71.9% versus 64.9% in PET2-negative patients (HR=0.720 [95% CI: 0.40–1.29], P=0.268), and 40.0% versus 25.0% in PET2-positive patients (HR=0.923 [95% CI: 0.23–3.72], P=0.910); however, numbers of patients were low in the PET2-positive, aged ≥60 years subgroup in the A+AVD arm (n=5) and the ABVD arm (n=8) (Online Supplementary Table S2). For both older and younger cHL patients, PFS rates were higher in PET2-negative versus PET2-positive patients within each study arm (Online Supplementary Table S2).
Per protocol, OS was assessed at the time of the pri- mary analysis (median follow-up 28 months) and the final analysis will be performed once 112 events have occurred in the entire study. Among older patients, 15 patients in the A+AVD arm and 17 in the ABVD arm had died as of the April 20, 2017 data cut. Data on salvage therapy are not available.
Safety
A total of 181 older patients were evaluable for safety (A+AVD: n=83, ABVD: n=98). Older patients received a median of six cycles of treatment across both treatment arms. In the A+AVD arm, 80% of older patients required one or more dose modification of brentuximab vedotin: dose reduction, 31%; dose held, 5%; dose delayed, 61%; brentuximab vedotin discontinued, 20%. The mean rela- tive dose intensity in older patients for brentuximab vedotin was 92%; relative dose intensities in the A+AVD versus ABVD arms for doxorubicin were 97% versus 97%; for vinblastine 93% versus 93%; and for dacarbazine 98% versus 96% (Online Supplementary Table S3). In the ABVD arm, 71% of older patients required one or more dose modification of bleomycin: dose reduction, 9%; dose held, 4%; dose interrupted, 1%; dose delayed, 49%; bleomycin discontinued, 28%. The mean relative dose intensity for bleomycin was 88.7% (Online Supplementary Table S3).
Overall, the incidences of grade ≥3 treatment-emergent adverse events were higher in older patients than in younger patients (Table 3). Within both age groups, there was a higher incidence of any-grade pulmonary-related events in the ABVD arm than in the A+AVD arm. In older patients, a total of eight deaths occurred on-study (within 30 days of the last dose of frontline treatment), which yielded a treatment-related mortality rate of 4.4% (8/181; 3/83 [3.6%] in the A+AVD arm and 5/98 [5.1%] in the ABVD arm). Of these eight deaths, three occurred in the A+AVD arm (due to hemophagocytic lymphohistiocyto- sis, multiple organ dysfunction syndrome, and myocardial infarction [each, n=1]), none of which was associated with pulmonary toxicity (Online Supplementary Table S4). The remaining five deaths occurred in the ABVD arm (due to pneumonia [n=2], interstitial lung disease [n=1], respiratory disorder [n=1], and cardiac arrest [n=1]). Treatment-related pulmonary-related toxicity was associated with three of these five deaths in the ABVD arm, occurring in patients aged 78, 80, and 83 years, and could not be ruled out as having a causal relationship with the other two deaths.
The incidence of grade ≥3 neutropenia was higher in the A+AVD arm than in the ABVD arm in older patients (70% vs. 59%). The incidence of any-grade febrile neutropenia
1088
haematologica | 2022; 107(5)