Page 83 - Haematologica May 2022
P. 83
Analysis of older cHL patients in ECHELON-1
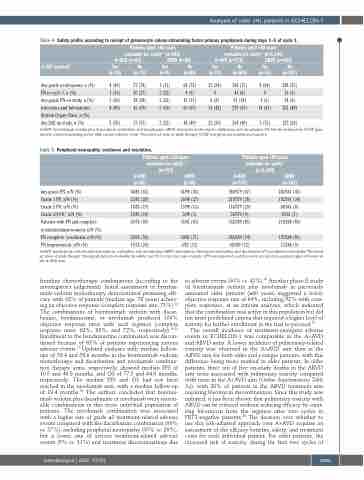
Table 4. Safety profile according to receipt of granulocyte colony-stimulating factor primary prophylaxis during days 1–5 of cycle 1.
Patients aged ≥60 years evaluable for safety* (n=181) A+AVD (n=83) ABVD (n=98)
Patients aged <60 years evaluable for safety* (n=1,140)
G-CSF received†
Any-grade neutropenia, n (%) FNincycle1,n(%) Any-gradeFNonstudy,n(%) Infections and Infestations System Organ Class, n (%)
Any SAE on study, n (%)
Yes (n=10)
4 (40) 1(10) 3(30) 8 (80)
5 (50)
No Yes (n=73) (n=9)
No (n=89)
64 (72)
8(9) 0 41(8) 0 16(3)
A+AVD (n=579)
ABVD (n=561)
Yes (n=73)
No (n=506)
Yes (n=34)
No (n=527)
57 (78) 20(27) 28(38) 43 (59)
53 (73)
1 (11) 2(22) 2(22) 5 (56)
2 (22)
25 (34)
368 (73)
8 (24)
288 (55)
15(17) 60 (67)
44 (49)
6(8) 91(18) 1(3) 34(6) 31 (42) 279 (55) 14 (41) 252 (48)
22 (30) 204 (40) 5 (15) 127 (24)
A+AVD: brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; FN: febrile neutropenia; G-CSF: gran- ulocyte colony-stimulating factor; SAE: serious adverse event. *Received ≥1 dose of study therapy. †G-CSF was given per institutional practice.
Table 5. Peripheral neuropathy: incidence and resolution.
Patients aged ≥60 years evaluable for safety* (n=181)
Patients aged <60 years evaluable for safety* (n=1,140)
Any-grade PN, n/N (%)
Grade 1 PN, n/N (%)
Grade 2 PN, n/N (%)
Grade 3/4 PN,† n/N (%)
Patients with PN and complete resolution/improvement, n/N (%) PN complete resolution, n/N (%)
PN improvement, n/N (%)
A+AVD (n=83)
54/83 (65) 23/83 (28) 16/83 (19) 15/83 (18) 43/54 (80)
30/54 (56)
13/54 (24)
ABVD (n=98)
42/98 (43) 26/98 (27) 13/98 (13) 3/98 (3) 35/42 (83)
30/42 (71) 5/42 (12)
A+AVD (n=579)
389/579 (67) 219/579 (38) 114/579 (20) 56/579 (9) 332/389 (85)
286/389 (74) 46/389 (12)
ABVD (n=561)
244/561 (43) 192/561 (34) 44/561 (8) 8/561 (1) 210/244 (86)
197/244 (81) 13/244 (5)
A+AVD:brentuximab vedotin plus doxorubicin,vinblastine,and dacarbazine;ABVD:doxorubicin,bleomycin,vinblastine,and dacarbazine;PN:peripheral neuropathy.*Received ≥1 dose of study therapy.†Among all patients evaluable for safety (n=1,321),only one case of grade 4 PN was reported,and this event occurred in a patient aged <60 years in the A+AVD arm.
frontline chemotherapy combinations (according to the investigator’s judgement). Initial assessment of brentux- imab vedotin monotherapy demonstrated promising effi- cacy with 92% of patients (median age, 78 years) achiev- ing an objective response (complete response rate: 73%).14 The combinations of brentuximab vedotin with dacar- bazine, bendamustine, or nivolumab produced 100% objective response rates with each regimen (complete response rates: 62%, 88%, and 72%, respectively).16,26 Enrollment to the bendamustine combination was discon- tinued because of 65% of patients experiencing serious adverse events.16 Updated analyses with median follow- ups of 59.4 and 58.6 months in the brentuximab vedotin monotherapy and dacarbazine and nivolumab combina- tion therapy arms, respectively, showed median PFS of 10.5 and 46.8 months, and OS of 77.5 and 64.0 months, respectively. The median PFS and OS had not been reached in the nivolumab arm, with a median follow-up of 19.4 months.25 The authors concluded that brentux- imab vedotin plus dacarbazine or nivolumab were reason- able combinations in this more unfit/frail population of patients. The nivolumab combination was associated with a higher rate of grade ≥3 treatment-related adverse events compared with the dacarbazine combination (60% vs. 37%), including peripheral neuropathy (35% vs. 26%), but a lower rate of serious treatment-related adverse events (5% vs. 11%) and treatment discontinuations due
to adverse events (30% vs. 42%).25 Another phase II study of brentuximab vedotin plus nivolumab in previously untreated older patients (≥60 years) suggested a lower objective response rate of 64%, including 52% with com- plete responses, at an interim analysis, which indicated that the combination was active in this population but did not meet predefined criteria that required a higher level of activity for further enrollment in the trial to proceed.27
The overall incidence of treatment-emergent adverse events in ECHELON-1 was comparable in the A+AVD and ABVD arms. A lower incidence of pulmonary-related toxicity was observed in the A+AVD arm than in the ABVD arm for both older and younger patients, with this difference being more marked in older patients. In older patients, three out of five on-study deaths in the ABVD arm were associated with pulmonary toxicity compared with none in the A+AVD arm (Online Supplementary Table S4), with 28% of patients in the ABVD treatment arm requiring bleomycin discontinuation. Since this study was initiated, it has been shown that pulmonary toxicity with ABVD can be reduced without reducing efficacy by omit- ting bleomycin from the regimen after two cycles in PET2-negative patients.28 The decision over whether to use this risk-adapted approach over A+AVD requires an assessment of the efficacy benefits, safety, and treatment costs for each individual patient. For older patients, the increased risk of toxicity, during the first two cycles of
haematologica | 2022; 107(5)
1091